Treatment of severe pressure ulcers with protein-enriched filtered platelet-rich plasma (PEFPRP): a possible management
- PMID: 38288245
- PMCID: PMC10823015
- DOI: 10.3389/fbioe.2023.1279149
Treatment of severe pressure ulcers with protein-enriched filtered platelet-rich plasma (PEFPRP): a possible management
Abstract
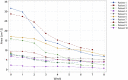
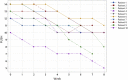
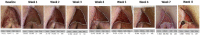
Background: Biological dressings with non-transfusion blood components are among the treatments available for pressure ulcers (PUs). Biological dressings contain active concentrated pro-regenerative molecules that can modify and switch off local inflammatory pathways. This re-establishes the physiological homing, which results in healing. In our study, we used a biological component obtained by ultrafiltration of plasma-platelet concentrate: protein-enriched filtered platelet-rich plasma (PEFPRP) with a higher platelet and higher plasma protein concentration. We tested whether treatment with PEFPRP could improve healing in advanced-stage pressure ulcers with a large surface area. All the patients in this study had a surgical indication but were not able to undergo surgery for various reasons. Materials and methods: Ten patients with severe neurological disability and advanced-stage sacral pressure ulcers were treated with allogenic PEFPRP. The mean lesion surface area at T0 was 13.4 cm2 ( ± 9.8 SD). PEFPRP was derived from allogenic plasma-platelet apheresis that had been pre-ultrafiltered with a ProSmart™ filter (Medica, Italy) to obtain a concentration after filtration of the plasma protein (12-16 g/dL) and platelet (1-1.2 x 106 microL). Results and Conclusion: All cases showed a reduction in the surface area of the pressure ulcer and in the Pressure Ulcer Scale for Healing (PUSH) score. The mean reduction values at week 6 were as follows: -52% for surface area and -21% for PUSH. Rapid wound healing is fundamental to avoid infections and improve patients' quality of life. This blood component builds new tissue by creating a new extracellular matrix. This, in turn, promotes rapid restoration of the three-dimensional structure of the tissue necessary for healing deeper wounds. PEFPRP shrinks the PU and improves its morphological features (reducing undermining and boosting granulation tissue). PEFPRP also promotes tissue restoration, obtaining an optimal scar. It is a safe and feasible treatment, and these preliminary results support the use of PEFPRP in the treatment of pressure ulcers. PEFPRP dressings could be integrated in the standard treatment of advanced-stage PU.
Keywords: dressing; neurologic patient; plasma proteins; pressure ulcers; protein-enriched filtered platelet-rich plasma (PEFPRP).
Copyright © 2024 Mazzucco, Balbo, Zingarelli, Desilvestri, Marchioni, Perrero, Pollis and Varvello.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

