Exploring nasopharyngeal microbiota profile in children affected by SARS-CoV-2 infection
- PMID: 38289047
- PMCID: PMC10913489
- DOI: 10.1128/spectrum.03009-23
Exploring nasopharyngeal microbiota profile in children affected by SARS-CoV-2 infection
Abstract
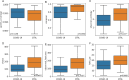
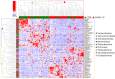
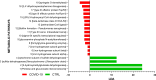
The relationship between COVID-19 and nasopharyngeal (NP) microbiota has been investigated mainly in the adult population. We explored the NP profile of children affected by COVID-19, compared to healthy controls (CTRLs). NP swabs of children with COVID-19, collected between March and September 2020, were investigated at the admission (T0), 72 h to 7 days (T1), and at the discharge (T2) of the patients. NP microbiota was analyzed by 16S rRNA targeted-metagenomics. Data from sequencing were investigated by QIIME 2.0 and PICRUSt 2. Multiple machine learning (ML) models were exploited to classify patients compared to CTRLs. The NP microbiota of COVID-19 patients (N = 71) was characterized by reduction of α-diversity compared to CTRLs (N = 59). The NP microbiota of COVID-19 cohort appeared significantly enriched in Streptococcus, Haemophilus, Staphylococcus, Veillonella, Enterococcus, Neisseria, Moraxella, Enterobacteriaceae, Gemella, Bacillus, and reduced in Faecalibacterium, Akkermansia, Blautia, Bifidobacterium, Ruminococcus, and Bacteroides, compared to CTRLs (FDR < 0.001). Exploiting ML models, Enterococcus, Pseudomonas, Streptococcus, Capnocytopagha, Tepidiphilus, Porphyromonas, Staphylococcus, and Veillonella resulted as NP microbiota biomarkers, in COVID-19 patients. No statistically significant differences were found comparing the NP microbiota profile of COVID-19 patients during the time-points or grouping patients on the basis of high, medium, and low viral load (VL). This evidence provides specific pathobiont signatures of the NP microbiota in pediatric COVID-19 patients, and the reduction of anaerobic protective commensals. Our data suggest that the NP microbiota may have a specific disease-related signature since infection onset without changes during disease progression, regardless of the SARS-CoV-2 VL.
Importance: Since the beginning of pandemic, we know that children are less susceptible to severe COVID-19 disease. A potential role of the nasopharyngeal (NP) microbiota has been hypothesized but to date, most of the studies have been focused on adults. We studied the NP microbiota modifications in children affected by SARS-CoV-2 infection showing a specific NP microbiome profile, mainly composed by pathobionts and almost missing protective anaerobic commensals. Moreover, in our study, specific microbial signatures appear since the first days of infection independently from SARS-CoV-2 viral load.
Keywords: COVID-19; SARS-CoV-2; children; nasopharyngeal microbiota; respiratory tract.
Conflict of interest statement
Authors G.M. and V.G. are employed by GenomeUp SRL, Viale Pasteur, Rome, Italy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Hasegawa K, Mansbach JM, Ajami NJ, Espinola JA, Henke DM, Petrosino JF, Piedra PA, Shaw CA, Sullivan AF, Camargo CA, the MARC-35 Investigators . 2016. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J 48:1329–1339. doi:10.1183/13993003.00152-2016 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

