Comparative analysis of Porphyromonas gingivalis A7436 and ATCC 33277 strains reveals differences in the expression of heme acquisition systems
- PMID: 38289063
- PMCID: PMC10913741
- DOI: 10.1128/spectrum.02865-23
Comparative analysis of Porphyromonas gingivalis A7436 and ATCC 33277 strains reveals differences in the expression of heme acquisition systems
Abstract
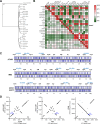
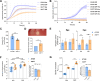
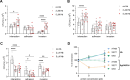
Porphyromonas gingivalis strains exhibit different phenotypes in vitro, different virulence potential in animal models, and different associations with human diseases, with strains classified as virulent/more virulent (e.g., A7436 and W83) or as less virulent/avirulent (e.g., ATCC 33277). In this study, we comparatively analyzed the A7436 and ATCC 33277 strains to better understand their variability. Global gene expression analysis in response to heme and iron limitation revealed more pronounced differences in the A7436 than in the ATCC 33277 strain; however, in both strains, the largest changes were observed in genes encoding hypothetical proteins, genes whose products participate in energy metabolism, and in genes encoding proteins engaged in transport and binding proteins. Our results confirmed that variability between P. gingivalis strains is due to differences in the arrangement of their genomes. Analysis of gene expression of heme acquisition systems demonstrated that not only the availability of iron and heme in the external environment but also the ability to store iron intracellularly can influence the P. gingivalis phenotype. Therefore, we assume that differences in virulence potential may also be due to differences in the production of systems involved in iron and heme acquisition, mainly the Hmu system. In addition, our study showed that hemoglobin, in a concentration-dependent manner, differentially influences the virulence potential of P. gingivalis strains. We conclude that iron and heme homeostasis may add to the variability observed between P. gingivalis strains.
Importance: Periodontitis belongs to a group of multifactorial diseases, characterized by inflammation and destruction of tooth-supporting tissues. P. gingivalis is one of the most important microbial factors involved in the initiation and progression of periodontitis. To survive in the host, the bacterium must acquire heme as a source of iron and protoporphyrin IX. P. gingivalis strains respond differently to changing iron and heme concentrations, which may be due to differences in the expression of systems involved in iron and heme acquisition. The ability to accumulate iron intracellularly, being different in more and less virulent P. gingivalis strains, may influence their phenotypes, production of virulence factors (including proteins engaged in heme acquisition), and virulence potential of this bacterium.
Keywords: HmuY; IhtB; Porphyromonas gingivalis; gingipain; heme; heme acquisition; hemoglobin; iron; strain variation; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

