Characterization of the resistome and predominant genetic lineages of Gram-positive bacteria causing keratitis
- PMID: 38289077
- PMCID: PMC10916405
- DOI: 10.1128/aac.01247-23
Characterization of the resistome and predominant genetic lineages of Gram-positive bacteria causing keratitis
Abstract
Bacterial keratitis is a vision-threatening infection mainly caused by Gram-positive bacteria (GPB). Antimicrobial therapy is commonly empirical using broad-spectrum agents with efficacy increasingly compromised by the emergence of antimicrobial resistance. We used a combination of phenotypic tests and genome sequencing to identify the predominant lineages of GPB causing keratitis and to characterize their antimicrobial resistance patterns. A total of 161 isolates, including Staphylococcus aureus (n = 86), coagulase-negative staphylococci (CoNS; n = 34), Streptococcus spp. (n = 34), and Enterococcus faecalis (n = 7), were included. The population of S. aureus isolates consisted mainly of clonal complex 5 (CC5) (30.2%). Similarly, the population of Staphylococcus epidermidis was homogenous with most of them belonging to CC2 (78.3%). Conversely, the genetic population of Streptococcus pneumoniae was highly diverse. Resistance to first-line antibiotics was common among staphylococci, especially among CC5 S. aureus. Methicillin-resistant S. aureus was commonly resistant to fluoroquinolones and azithromycin (78.6%) and tobramycin (57%). One-third of the CoNS were resistant to fluoroquinolones and 53% to azithromycin. Macrolide resistance was commonly caused by erm genes in S. aureus, mphC and msrA in CoNS, and mefA and msr(D) in streptococci. Aminoglycoside resistance in staphylococci was mainly associated with genes commonly found in mobile genetic elements and that encode for nucleotidyltransferases like ant(4')-Ib and ant(9)-Ia. Fluroquinolone-resistant staphylococci carried from 1 to 4 quinolone resistance-determining region mutations, mainly in the gyrA and parC genes. We found that GPB causing keratitis are associated with strains commonly resistant to first-line topical therapies, especially staphylococcal isolates that are frequently multidrug-resistant and associated with major hospital-adapted epidemic lineages.
Keywords: Gram-positive bacteria; MRSA; antimicrobial resistance; genomics; keratitis; resistome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

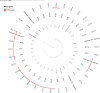
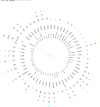
Similar articles
-
Microbiology of Eye Infections at the Massachusetts Eye and Ear: An 8-Year Retrospective Review Combined With Genomic Epidemiology.Am J Ophthalmol. 2023 Nov;255:43-56. doi: 10.1016/j.ajo.2023.06.016. Epub 2023 Jun 19. Am J Ophthalmol. 2023. PMID: 37343741 Free PMC article.
-
[Distribution and antibiotic resistance analysis of Gram positive cocci in bloodstream infections in a hospital in Inner Mongolia].Zhonghua Yu Fang Yi Xue Za Zhi. 2024 Aug 6;58(8):1242-1246. doi: 10.3760/cma.j.cn112150-20231120-00354. Zhonghua Yu Fang Yi Xue Za Zhi. 2024. PMID: 39142895 Chinese.
-
[Investigation of macrolide, lincosamide and streptogramin B resistance in Staphylococcus aureus strains isolated from clinical samples by phenotypical and genotypical methods].Mikrobiyol Bul. 2015 Jan;49(1):1-14. Mikrobiyol Bul. 2015. PMID: 25706726 Turkish.
-
Literature review on the distribution characteristics and antimicrobial resistance of bacterial pathogens in neonatal sepsis.J Matern Fetal Neonatal Med. 2022 Mar;35(5):861-870. doi: 10.1080/14767058.2020.1732342. Epub 2020 Feb 26. J Matern Fetal Neonatal Med. 2022. PMID: 32102584 Review.
-
Antibiotic Resistance in the Treatment of Staphylococcus aureus Keratitis: a 20-Year Review.Cornea. 2015 Jun;34(6):698-703. doi: 10.1097/ICO.0000000000000431. Cornea. 2015. PMID: 25811722 Free PMC article. Review.
Cited by
-
In Vitro Activity of Bacteriophages Against Ocular Methicillin-resistant S. aureus Isolates Collected in the US.Ophthalmol Ther. 2025 May;14(5):897-909. doi: 10.1007/s40123-025-01113-2. Epub 2025 Mar 12. Ophthalmol Ther. 2025. PMID: 40072828 Free PMC article.
-
An exploration of the ocular mysteries linking nanoparticles to the patho-therapeutic effects against keratitis.J Nanobiotechnology. 2025 Mar 6;23(1):184. doi: 10.1186/s12951-025-03230-3. J Nanobiotechnology. 2025. PMID: 40050881 Free PMC article. Review.
-
Synthetic lincosamides iboxamycin and cresomycin are active against ocular multidrug-resistant methicillin-resistant Staphylococcus aureus carrying erm genes.J Glob Antimicrob Resist. 2024 Dec;39:144-148. doi: 10.1016/j.jgar.2024.09.001. Epub 2024 Sep 16. J Glob Antimicrob Resist. 2024. PMID: 39293511 Free PMC article.
References
-
- Bourne RRA, Jonas JB, Bron AM, Cicinelli MV, Das A, Flaxman SR, Friedman DS, Keeffe JE, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Peto T, Saadine J, Silvester AJ, Tahhan N, Taylor HR, Varma R, Wong TY, Resnikoff S, Vision Loss Expert Group of the Global Burden of Disease Study . 2018. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. Br J Ophthalmol 102:575–585. doi:10.1136/bjophthalmol-2017-311258 - DOI - PMC - PubMed
-
- Lin A, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Varu DM, Musch DC, Dunn SP, Mah FS, American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel . 2019. Bacterial keratitis preferred practice pattern. Ophthalmology 126:P1–P55. doi:10.1016/j.ophtha.2018.10.018 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

