Borreliella burgdorferi factor H-binding proteins are not required for serum resistance and infection in mammals
- PMID: 38289123
- PMCID: PMC10929407
- DOI: 10.1128/iai.00529-23
Borreliella burgdorferi factor H-binding proteins are not required for serum resistance and infection in mammals
Abstract
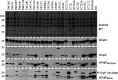
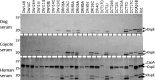
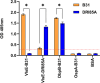
The causative agent of Lyme disease (LD), Borreliella burgdorferi, binds factor H (FH) and other complement regulatory proteins to its surface. B. burgdorferi B31 (type strain) encodes five FH-binding proteins (FHBPs): CspZ, CspA, and the OspE paralogs OspEBBN38, OspEBBL39, and OspEBBP38. This study assessed potential correlations between the production of individual FHBPs, FH-binding ability, and serum resistance using a panel of infectious B. burgdorferi clonal populations recovered from dogs. FHBP production was assessed in cultivated spirochetes and by antibody responses in naturally infected humans, dogs, and eastern coyotes (wild canids). FH binding specificity and sensitivity to dog and human serum were also assessed and compared. No correlation was observed between the production of individual FHBPs and FH binding with serum resistance, and CspA was determined to not be produced in animals. Notably, one or more clones isolated from dogs lacked CspZ or the OspE proteins (a finding confirmed by genome sequence determination) and did not bind FH derived from canines. The data presented do not support a correlation between FH binding and the production of individual FHBPs with serum resistance and infectivity. In addition, the limited number and polymorphic nature of cp32s in B. burgdorferi clone DRI85A that were identified through genome sequencing suggest no strict requirement for a defined set of these replicons for infectivity. This study reveals that the immune evasion mechanisms employed by B. burgdorferi are diverse, complex, and yet to be fully defined.
Keywords: CspA; CspZ; Lyme disease; OspE; complement evasion; cp32; dogs; factor H.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

