Amidinatotetrylenes Donor Functionalized on Both N Atoms: Structures and Coordination Chemistry
- PMID: 38289155
- PMCID: PMC10865366
- DOI: 10.1021/acs.inorgchem.3c04135
Amidinatotetrylenes Donor Functionalized on Both N Atoms: Structures and Coordination Chemistry
Abstract
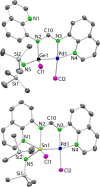
E(hmds)(bqfam) (E = Ge (1a), Sn (1b); hmds = N(SiMe3)2, bqfam = N,N'-bis(quinol-8-yl)formamidinate), which are amidinatotetrylenes equipped with quinol-8-yl fragments on the amidinate N atoms, have been synthesized from the formamidine Hbqfam and Ge(hmds)2 or SnCl(hmds). Both 1a and 1b are fluxional in solution at room temperature, as the E atom oscillates from being attached to the two amidinate N atoms to being chelated by an amidinate N atom and its closest quinolyl N atom (both situations are similarly stable according to density functional theory calculations). The hmds group of 1a and 1b is still reactive and the deprotonation of another equivalent of Hbqfam can be achieved, allowing the formation of the homoleptic derivatives E(bqfam)2 (E = Ge, Sn). The reactions of 1a and 1b with [AuCl(tht)] (tht = tetrahydrothiophene), [PdCl2(MeCN)2], [PtCl2(cod)] (cod = cycloocta-1,5-diene), [Ru3(CO)12] and [Co2(CO)8] have been investigated. The gold(I) complexes [AuCl{κE-E(hmds)(bqfam)}] (E = Ge, Sn) have a monodentate κE-tetrylene ligand and display fluxional behavior in solution the same as that of 1a and 1b. However, the palladium(II) and platinum(II) complexes [MCl{κ3E,N,N'-ECl(hmds)(bqfam)}] (M = Pd, Pt; E = Ge, Sn) contain a κ3E,N,N'-chloridotetryl ligand that arises from the insertion of the tetrylene E atom into an M-Cl bond and the coordination of an amidinate N atom and its closest quinolyl N atom to the metal center. Finally, the binuclear ruthenium(0) and cobalt(0) complexes [Ru2{μE-κ3E,N,N'-E(hmds)(bqfam)}(CO)6] and [Co2{μE-κ3E,N,N'-E(hmds)(bqfam)}(μ-CO)(CO)4] (E = Ge, Sn) have a related κ3E,N,N'-tetrylene ligand that bridges two metal atoms through the E atom. For the κ3E,N,N'-metal complexes, the quinolyl fragment not attached to the metal is pendant in all the germanium compounds but, for the tin derivatives, is attached to (in the Pd and Pt complexes) or may interact with (in the Ru2 and Co2 complexes) the tin atom.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
-
-
For some reviews on general chemistry of HTs (they might include coordination chemistry), see:
- Zhang Y.; Wu L.; Wang H. Application of N-heterocyclic silylenes in low-valent group 13, 14 and 15 chemistry. Coord. Chem. Rev. 2023, 477, 214942–214961. 10.1016/j.ccr.2022.214942. - DOI
- Yao S.; Saddington A.; Xiong Y.; Driess M. Chelating Bis-silylenes As Powerful Ligands To Enable Unusual Low-Valent Main-Group Element Functions. Acc. Chem. Res. 2023, 56, 475–488. 10.1021/acs.accounts.2c00763. - DOI - PubMed
- Wang L.; Li Y.; Li Z.; Kira M. Isolable Silylenes and their Diverse Reactivity. Coord. Chem. Rev. 2022, 457, 214413–214431. 10.1016/j.ccr.2022.214413. - DOI
- Se N.; Khan S. Heavier Tetrylenes as Single Site Catalysts. Chem.—Asian J. 2021, 16, 705–719. 10.1002/asia.202100038. - DOI - PubMed
- Dasgupta R.; Khan S. N-Heterocyclic Germylenes and Stannylenes: Synthesis, Reactivity and Catalytic Application in a Nutshell. Adv. Organomet. Chem. 2020, 74, 105–152. 10.1016/bs.adomc.2020.04.001. - DOI
- Fujimori S.; Inoue S. Small Molecule Activation by Two-Coordinate Acyclic Silylenes. Eur. J. Inorg. Chem. 2020, 3131–3142. 10.1002/ejic.202000479. - DOI - PMC - PubMed
- Khan S.; Roesky H. W. Carbene-Stabilized Exceptional Silicon Halides. Chem.—Eur. J. 2019, 25, 1636–1648. 10.1002/chem.201801672. - DOI - PubMed
- Hadlington T.; Driess M.; Jones C. Low-Valent Group 14 Element Hydride Chemistry: Towards Catalysis. Chem. Soc. Rev. 2018, 47, 4176–4197. 10.1039/C7CS00649G. - DOI - PubMed
- Rivard E. Group 14 Inorganic Hydrocarbon Analogues. Chem. Soc. Rev. 2016, 45, 989–1003. 10.1039/C5CS00365B. - DOI - PubMed
- Marschner C. Silylated Group 14 Ylenes: An Emerging Class of Reactive Compounds. Eur. J. Inorg. Chem. 2015, 2015, 3805–3820. 10.1002/ejic.201500495. - DOI
- Prabusankar G.; Sathyanarayana A.; Suresh P.; Babu C. N.; Srinivas K.; Metla B. P. R. N-Heterocyclic Carbene Supported Heavier Group 14 Elements: Recent Progress and Challenges. Coord. Chem. Rev. 2014, 269, 96–133. 10.1016/j.ccr.2014.01.036. - DOI
- Izod K. Heavier group 14 complexes with anionic P-donor ligands Coord. Chem. Rev. 2013, 257, 924–945. 10.1016/j.ccr.2013.01.004. - DOI
- Xiong Y.; Yao S.; Driess M. Chemical Tricks To Stabilize Silanones and Their Heavier Homologues with E = O Bonds (E = Si–Pb): From Elusive Species to Isolable Building Blocks. Angew. Chem., Int. Ed. 2013, 52, 4302–4311. 10.1002/anie.201209766. - DOI - PubMed
- Asay M.; Jones C.; Driess M. N-Heterocyclic Carbene Analogues with Low-Valent Group 13 and Group 14 Elements: Syntheses, Structures, and Reactivities of a New Generation of Multitalented Ligands. Chem. Rev. 2011, 111, 354–396. 10.1021/cr100216y. - DOI - PubMed
- Mandal S. K.; Roesky H. W. Interstellar molecules: guides for new chemistry. Chem. Commun. 2010, 46, 6016–6041. 10.1039/c0cc01003k. - DOI - PubMed
- Mizuhata Y.; Sasamori T.; Tokitoh N. Stable Heavier Carbene Analogues. Chem. Rev. 2009, 109, 3479–2511. 10.1021/cr900093s. - DOI - PubMed
-
-
-
For some reviews more focused on the coordination chemistry of HTs, see:
- Cabeza J. A.; García-Álvarez P. Tetrelanes versus Tetrylenes as Precursors to Transition Metal Complexes Featuring Tridentate PEP Tetryl Ligands (E=Si, Ge, Sn). Chem. Eur. J. 2023, 29, e202203096.10.1002/chem.202381861. - DOI - PubMed
- Lee V. L. Schrock-Type Silylidenes and Germylidenes Found Among the Silylene and Germylene Complexes of the Early and Mid-Transition Metals. Eur. J. Inorg. Chem. 2022, 2022, e20220017510.1002/ejic.202200175. - DOI
- Somerville R. J.; Campos J. Cooperativity in Transition Metal Tetrylene Complexes. Eur. J. Inorg. Chem. 2021, 2021, 3488–3498. 10.1002/ejic.202100460. - DOI - PMC - PubMed
- Gosh M.; Khan S. N-Heterocyclic silylenes in coinage metal chemistry: an account of recent advances. Dalton. Trans. 2021, 50, 10674–10688. 10.1039/D1DT01955D. - DOI - PubMed
- Tacke R.; Ribbeck T. Bis(amidinato)- and bis(guanidinato)- silylenes and silylenes with one sterically demanding amidinato or guanidinato ligand: synthesis and reactivity. Dalton Trans. 2017, 46, 13628–13659. 10.1039/C7DT01297G. - DOI - PubMed
- Álvarez-Rodríguez L.; Cabeza J. A.; García-Álvarez P.; Polo D. The transition-metal chemistry of amidinatosilylenes, -germylenes and -stannylenes. Coord. Chem. Rev. 2015, 300, 1–28. 10.1016/j.ccr.2015.04.008. - DOI
- Baumgartner J.; Marschner C. Coordination of Non-Stabilized Germylenes, Stannylenes, and Plumbylenes to Transition Metals. Rev. Inorg. Chem. 2014, 34, 119–152. 10.1515/revic-2013-0014. - DOI
- Blom B.; Stoelzel M.; Driess M. New Vistas in N-Heterocyclic Silylene (NHSi) Transition-Metal Coordination Chemistry: Syntheses, Structures and Reactivity towards Activation of Small Molecules. Chem.—Eur. J. 2013, 19, 40–62. 10.1002/chem.201203072. - DOI - PubMed
- Waterman R.; Hayes P. G.; Tilley T. D. Development and Chemical Reactivity of Transition-Metal Silylene Complexes. Acc. Chem. Res. 2007, 40, 712–719. 10.1021/ar700028b. - DOI - PubMed
- Lappert M. F.; Rowe R. S. The Role of Group 14 Element Carbene Analogues in Transition Metal Chemistry. Coord. Chem. Rev. 1990, 100, 267–292. 10.1016/0010-8545(90)85012-H. - DOI
-
-
-
For some reviews more focused on HT transition-metal complexes in catalysis, see:
- Cabeza J. A.; García-Álvarez P. Cyclometallation of Heavier Tetrylenes: Reported Complexes and Applications in Catalysis. Eur. J. Inorg. Chem. 2021, 2021, 3315–3326. 10.1002/ejic.202100430. - DOI
- Zhou Y.-P.; Driess M. Isolable Silylene Ligands Can Boost Efficiencies and Selectivities in Metal-Mediated Catalysis. Angew. Chem., Int. Ed. 2019, 58, 3715–3728. 10.1002/anie.201811088. - DOI - PubMed
- Raoufmoghaddam S.; Zhou Y.-P.; Wang Y.; Driess M. N-heterocyclic silylenes as powerful steering ligands in catalysis. J. Organomet. Chem. 2017, 829, 2–10. 10.1016/j.jorganchem.2016.07.014. - DOI
- Blom B.; Gallego D.; Driess M. N-heterocyclic silylene complexes in catalysis: new frontiers in an emerging field. Inorg. Chem. Front. 2014, 1, 134–148. 10.1039/C3QI00079F. - DOI
-
-
-
For examples of catalytic transformations promoted by metal-complexes equipped with monodentate-ATs, see:
- Fan Q.; Du X.; Yang W.; Li Q.; Huang W.; Sun H.; Hinz A.; Li X. Effects of silylene ligands on the performance of carbonyl hydrosilylation catalyzed by cobalt phosphine complexes. Dalton Trans. 2023, 52, 6712–6721. 10.1039/D3DT00372H. - DOI - PubMed
- Hossain J.; Sai J. S.; Srinu T.; Parameswaran P.; Khan S. NHSi/NHGe-Supported Copper Halide and Pseudohalide Complexes: Synthesis and Application. Organometallics 2022, 41, 3706–3717. 10.1021/acs.organomet.2c00480. - DOI
- Parvin N.; Hossain J.; George A.; Parameswaran P.; Khan S. N-heterocyclic silylene stabilized monocordinated copper(I)–arene cationic complexes and their application in click chemistry. Chem. Commun. 2020, 56, 273–276. 10.1039/C9CC09115G. - DOI - PubMed
- Parvin N.; Mishra B.; George A.; Neralkar M.; Hossain J.; Parameswaran P.; Hotha S.; Khan S. N-Heterocyclic silylene/germylene ligands in Au(I) catalysis. Chem. Commun. 2020, 56, 7625–7628. 10.1039/D0CC03156A. - DOI - PubMed
- Paesch A. N.; Kreyenschmidt A.-K.; Herbst-Irmer R.; Stalke D. Side-Arm Functionalized Silylene Copper(I) Complexes in Catalysis. Inorg. Chem. 2019, 58, 7000–7009. 10.1021/acs.inorgchem.9b00629. - DOI - PubMed
- Qi X.; Sun H.; Li X.; Fuhr O.; Fenske D. Synthesis and catalytic activity of N-heterocyclic silylene (NHSi) cobalt hydride for Kumada coupling reactions. Dalton Trans. 2018, 47, 2581–2588. 10.1039/C7DT04155A. - DOI - PubMed
- Álvarez-Rodríguez L.; Cabeza J. A.; García-Álvarez P.; Pérez-Carreño E. Ruthenium Carbene Complexes Analogous to Grubbs-I Catalysts Featuring Germylenes as Ancillary Ligands. Organometallics 2018, 37, 3399–3406. 10.1021/acs.organomet.7b00905. - DOI
- Khoo S. S.; Jiajia C.; Yang M.-C.; Shan Y.-L.; Su M.-D.; So C.-W. Synthesis of a Dimeric Base-Stabilized Cobaltosilylene Complex for Catalytic C–H Bond Functionalization and C–C Bond Formation. Chem.—Eur. J. 2018, 24, 14329–14334. 10.1002/chem.201803410. - DOI - PubMed
- Álvarez-Rodríguez L.; Cabeza J. A.; Fernández-Colinas J. M.; García-Álvarez P.; Polo D. Amidinatogermylene Metal Complexes as Homogeneous Catalysts in Alcoholic Media. Organometallics 2016, 35, 2516–2523. 10.1021/acs.organomet.6b00426. - DOI
- Blom B.; Enthaler S.; Inoue S.; Irran E.; Driess M. Electron-Rich N-Heterocyclic Silylene (NHSi)–Iron Complexes: Synthesis, Structures, and Catalytic Ability of an Isolable Hydridosilylene–Iron Complex. J. Am. Chem. Soc. 2013, 135, 6703–6803. 10.1021/ja402480v. - DOI - PubMed
-
-
-
For examples of catalytic transformations promoted by metal complexes equipped with polydentate-ATs, see:
- Jia H.; Du S.; Xu C.; Mo Z. Hydrogenation of Olefins Catalyzed by a Cobalt(I) Hydride Complex with N-Heterocyclic Silylene. Eur. J. Inorg. Chem. 2023, 26, e20230008610.1002/ejic.202300086. - DOI
- Roque J. B.; Pabst T. P.; Chirik P. J. C(sp2)–H Activation with Bis(silylene)pyridine Cobalt(III) Complexes: Catalytic Hydrogen Isotope Exchange of Sterically Hindered C–H Bonds. ACS Catal. 2022, 12, 8877–8885. 10.1021/acscatal.2c02429. - DOI - PMC - PubMed
- Lücke M.-P.; Yao S.; Driess M. Boosting homogeneous chemoselective hydrogenation of olefins mediated by a bis(silylenyl)terphenyl-nickel(0) pre-catalyst. Chem. Sci. 2021, 12, 2909–2915. 10.1039/D0SC06471H. - DOI - PMC - PubMed
- Sun X.; Simler T.; Kraetschmer F.; Roesky P. W. Thermally Stable Rare-Earth Metal Complexes Supported by Chelating Silylene Ligands. Organometallics 2021, 40, 2100–2107. 10.1021/acs.organomet.1c00238. - DOI
- Li S.; Wang Y.; Yang W.; Li K.; Sun H.; Li X.; Fuhr O.; Fenske D. N2 Silylation Catalyzed by a Bis(silylene)-Based [SiCSi] Pincer Hydrido Iron(II) Dinitrogen Complex. Organometallics 2020, 39, 757–766. 10.1021/acs.organomet.0c00025. - DOI
- Arevalo R.; Pabst T. P.; Chirik P. J. C(sp2)–H Borylation of Heterocycles by Well-Defined Bis(silylene)pyridine Cobalt(III) Precatalysts: Pincer Modification, C(sp2)–H Activation, and Catalytically Relevant Intermediates. Organometallics 2020, 39, 2763–2773. 10.1021/acs.organomet.0c00382. - DOI - PMC - PubMed
- Qi X.; Zheng T.; Zhou J.; Dong Y.; Zuo X.; Li X.; Sun H.; Fuhr O.; Fenske D. Synthesis and Catalytic Activity of Iron Hydride Ligated with Bidentate N-Heterocyclic Silylenes for Hydroboration of Carbonyl Compounds. Organometallics 2019, 38, 268–277. 10.1021/acs.organomet.8b00700. - DOI
- Zhou Y.-P.; Mo Z.; Luecke M.-P.; Driess M. Stereoselective Transfer Semi-Hydrogenation of Alkynes to E-Olefins with N-Heterocyclic Silylene–Manganese Catalysts. Chem.—Eur. J. 2018, 24, 4780–4784. 10.1002/chem.201705745. - DOI - PubMed
- Bai Y.; Zhanga J.; Cui C. An arene-tethered silylene ligand enabling reversible dinitrogen binding to iron and catalytic silylation. Chem. Commun. 2018, 54, 8124–8127. 10.1039/C8CC03734E. - DOI - PubMed
- Mo Z.; Kostenko A.; Zhou Y.-P.; Yao S.; Driess M. Chelate Silylene–Silyl Ligand Can Boost Rhodium-Catalyzed C–H Bond Functionalization Reactions. Chem.—Eur. J. 2018, 24, 14608–14612. 10.1002/chem.201803089. - DOI - PubMed
- Cabeza J. A.; García-Álvarez P.; González-Álvarez L. Facile cyclometallation of a mesitylsilylene: synthesis and preliminary catalytic activity of iridium(III) and iridium(V) iridasilacyclopentenes. Chem. Commun. 2017, 53, 10275–10278. 10.1039/C7CC04832G. - DOI - PubMed
- Schmidt M.; Blom B.; Szilvási T.; Schomacker R.; Driess M. Improving the Catalytic Activity in the Rhodium-Mediated Hydroformylation of Styrene by a Bis(N-heterocyclic silylene) Ligand. Eur. J. Inorg. Chem. 2017, 2017, 1284–1291. 10.1002/ejic.201700148. - DOI
- Luecke M. P.; Porwai D.; Kostenko A.; Zhou Y.-P.; Yao S.; Keck M.; Limberg C.; Oestreich M.; Driess M. Bis(silylenyl)-substituted ferrocene-stabilized η6-arene iron(0) complexes: synthesis, structure and catalytic application. Dalton Trans. 2017, 46, 16412–16418. 10.1039/C7DT03301J. - DOI - PubMed
- Ren H.; Zhou Y.-P.; Bai Y.; Cui C.; Driess M. Cobalt-Catalyzed Regioselective Borylation of Arenes: N-Heterocyclic Silylene as an Electron Donor in the Metal-Mediated Activation of C–H Bonds. Chem.—Eur. J. 2017, 23, 5663–5667. 10.1002/chem.201605937. - DOI - PubMed
- Wang Y.; Kostenko A.; Yao S.; Driess M. Divalent Silicon-Assisted Activation of Dihydrogen in a Bis(N-heterocyclic silylene)xanthene Nickel(0) Complex for Efficient Catalytic Hydrogenation of Olefins. J. Am. Chem. Soc. 2017, 139, 13499–13506. 10.1021/jacs.7b07167. - DOI - PubMed
- Zhou Y.-P.; Raoufmoghaddam S.; Szilvási T.; Driess M. A Bis(silylene)-Substituted ortho-Carborane as a Superior Ligand in the Nickel-Catalyzed Amination of Arenes. Angew. Chem., Int. Ed. 2016, 55, 12868–12872. 10.1002/anie.201606979. - DOI - PubMed
- Metsänen T. T.; Gallego D.; Szilvási T.; Driess M.; Oestreich M. Peripheral mechanism of a carbonyl hydrosilylation catalysed by an SiNSi iron pincer complex. Chem. Sci. 2015, 6, 7143–7149. 10.1039/C5SC02855H. - DOI - PMC - PubMed
- Gallego D.; Inoue S.; Blom B.; Driess M. Highly Electron-Rich Pincer-Type Iron Complexes Bearing Innocent Bis(metallylene)pyridine Ligands: Syntheses, Structures, and Catalytic Activity. Organometallics 2014, 33, 6885–6897. 10.1021/om500966t. - DOI
- Gallego D.; Bruck A.; Irran E.; Meier F.; Kaupp M.; Driess M.; Hartwig J. F. From Bis(silylene) and Bis(germylene) Pincer-Type Nickel(II) Complexes to Isolable Intermediates of the Nickel-Catalyzed Sonogashira Cross-Coupling Reaction. J. Am. Chem. Soc. 2013, 135, 15617–15626. 10.1021/ja408137t. - DOI - PubMed
- Someya C. I.; Haberberger M.; Wang W.; Enthalter S.; Inoue S. Application of a Bis(silylene) Nickel Complex as Precatalyst in C–C Bond Formation Reactions. Chem. Lett. 2013, 42, 286–288. 10.1246/cl.2013.286. - DOI
- Wang W.; Inoue S.; Enthaler S.; Driess M. Bis(silylenyl)- and Bis(germylenyl)-Substituted Ferrocenes: Synthesis, Structure, and Catalytic Applications of Bidentate Silicon(II)–Cobalt Complexes. Angew. Chem., Int. Ed. 2012, 51, 6167–6171. 10.1002/anie.201202175. - DOI - PubMed
- Brück A.; Gallego D.; Wang W.; Irran E.; Driess M.; Hartwig J. F. Pushing the σ-Donor Strength in Iridium Pincer Complexes: Bis(silylene) and Bis(germylene) Ligands Are Stronger Donors than Bis(phosphorus(III)) Ligands. Angew. Chem., Int. Ed. 2012, 51, 11478–11482. 10.1002/anie.201205570. - DOI - PubMed
- Ahuja H.; Kaur H.; Arevalo R. Chemoselective C(sp)–H borylation of terminal alkynes catalyzed by a bis(N-heterocyclicsilylene) manganese complex. Inorg. Chem. Front. 2023, 10, 6067–6076. 10.1039/D3QI01033C. - DOI
-
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

