MuSCs and IPCs: roles in skeletal muscle homeostasis, aging and injury
- PMID: 38289345
- PMCID: PMC10828015
- DOI: 10.1007/s00018-023-05096-w
MuSCs and IPCs: roles in skeletal muscle homeostasis, aging and injury
Abstract
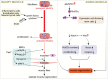
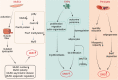
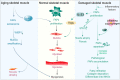
Skeletal muscle is a highly specialized tissue composed of myofibres that performs crucial functions in movement and metabolism. In response to external stimuli and injuries, a range of stem/progenitor cells, with muscle stem cells or satellite cells (MuSCs) being the predominant cell type, are rapidly activated to repair and regenerate skeletal muscle within weeks. Under normal conditions, MuSCs remain in a quiescent state, but become proliferative and differentiate into new myofibres in response to injury. In addition to MuSCs, some interstitial progenitor cells (IPCs) such as fibro-adipogenic progenitors (FAPs), pericytes, interstitial stem cells expressing PW1 and negative for Pax7 (PICs), muscle side population cells (SPCs), CD133-positive cells and Twist2-positive cells have been identified as playing direct or indirect roles in regenerating muscle tissue. Here, we highlight the heterogeneity, molecular markers, and functional properties of these interstitial progenitor cells, and explore the role of muscle stem/progenitor cells in skeletal muscle homeostasis, aging, and muscle-related diseases. This review provides critical insights for future stem cell therapies aimed at treating muscle-related diseases.
Keywords: Fibro-adipogenic progenitors; Interstitial progenitor cells; Muscle stem cells; Pericytes; Skeletal muscle.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

