A plasma membrane-associated form of the androgen receptor enhances nuclear androgen signaling in osteoblasts and prostate cancer cells
- PMID: 38289986
- PMCID: PMC10916501
- DOI: 10.1126/scisignal.adi7861
A plasma membrane-associated form of the androgen receptor enhances nuclear androgen signaling in osteoblasts and prostate cancer cells
Abstract
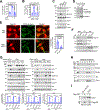
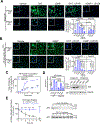
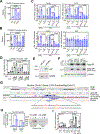
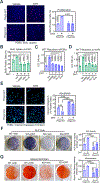
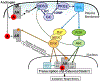
Androgen binding to the androgen receptor (AR) in the cytoplasm induces the AR to translocate to the nucleus, where it regulates the expression of target genes. Here, we found that androgens rapidly activated a plasma membrane-associated signaling node that enhanced nuclear AR functions. In murine primary osteoblasts, dihydrotestosterone (DHT) binding to a membrane-associated form of AR stimulated plasma membrane-associated protein kinase G type 2 (PKG2), leading to the activation of multiple kinases, including ERK. Phosphorylation of AR at Ser515 by ERK increased the nuclear accumulation and binding of AR to the promoter of Ctnnb1, which encodes the transcription factor β-catenin. In male mouse osteoblasts and human prostate cancer cells, DHT induced the expression of Ctnnb1 and CTNN1B, respectively, as well as β-catenin target genes, stimulating the proliferation, survival, and differentiation of osteoblasts and the proliferation of prostate cancer cells in a PKG2-dependent fashion. Because β-catenin is a master regulator of skeletal homeostasis, these results explain the reported male-specific osteoporotic phenotype of mice lacking PKG2 in osteoblasts and imply that PKG2-dependent AR signaling is essential for maintaining bone mass in vivo. Our results suggest that widely used pharmacological PKG activators, such as sildenafil, could be beneficial for male and estrogen-deficient female patients with osteoporosis but detrimental in patients with prostate cancer.
Conflict of interest statement
Figures



References
-
- Ahluwalia A, Hoa N, Ge L, Blumberg B, Levin ER, Mechanisms by Which Membrane and Nuclear ER Alpha Inhibit Adipogenesis in Cells Isolated From Female Mice. Endocrinology 161, (2020). - PubMed
-
- Thomas P, Membrane Androgen Receptors Unrelated to Nuclear Steroid Receptors. Endocrinology 160, 772–781 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

