mTORC1 controls murine postprandial hepatic glycogen synthesis via Ppp1r3b
- PMID: 38290087
- PMCID: PMC10977990
- DOI: 10.1172/JCI173782
mTORC1 controls murine postprandial hepatic glycogen synthesis via Ppp1r3b
Abstract
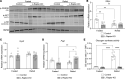
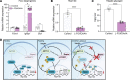
In response to a meal, insulin drives hepatic glycogen synthesis to help regulate systemic glucose homeostasis. The mechanistic target of rapamycin complex 1 (mTORC1) is a well-established insulin target and contributes to the postprandial control of liver lipid metabolism, autophagy, and protein synthesis. However, its role in hepatic glucose metabolism is less understood. Here, we used metabolomics, isotope tracing, and mouse genetics to define a role for liver mTORC1 signaling in the control of postprandial glycolytic intermediates and glycogen deposition. We show that mTORC1 is required for glycogen synthase activity and glycogenesis. Mechanistically, hepatic mTORC1 activity promotes the feeding-dependent induction of Ppp1r3b, a gene encoding a phosphatase important for glycogen synthase activity whose polymorphisms are linked to human diabetes. Reexpression of Ppp1r3b in livers lacking mTORC1 signaling enhances glycogen synthase activity and restores postprandial glycogen content. mTORC1-dependent transcriptional control of Ppp1r3b is facilitated by FOXO1, a well characterized transcriptional regulator involved in the hepatic response to nutrient intake. Collectively, we identify a role for mTORC1 signaling in the transcriptional regulation of Ppp1r3b and the subsequent induction of postprandial hepatic glycogen synthesis.
Keywords: Endocrinology; Glucose metabolism; Insulin signaling; Metabolism.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

