Early-life pulmonary viral infection leads to long-term functional and lower airway structural changes in the lungs
- PMID: 38290164
- PMCID: PMC11281791
- DOI: 10.1152/ajplung.00300.2023
Early-life pulmonary viral infection leads to long-term functional and lower airway structural changes in the lungs
Abstract
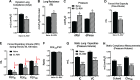
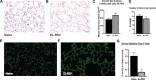
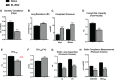
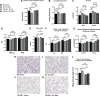
Early-life respiratory virus infections have been correlated with enhanced development of childhood asthma. In particular, significant numbers of respiratory syncytial virus (RSV)-hospitalized infants go on to develop lung disease. It has been suggested that early-life viral infections may lead to altered lung development or repair that negatively impacts lung function later in life. Our data demonstrate that early-life RSV infection modifies lung structure, leading to decreased lung function. At 5 wk postneonatal RSV infection, significant defects are observed in baseline pulmonary function test (PFT) parameters consistent with decreased lung function as well as enlarged alveolar spaces. Lung function changes in the early-life RSV-infected group continue at 3 mo of age. The altered PFT and structural changes induced by early-life RSV were mitigated in TSLPR-/- mice that have previously been shown to have reduced immune cell accumulation associated with a persistent Th2 environment. Importantly, long-term effects were demonstrated using a secondary RSV infection 3 mo following the initial early-life RSV infection and led to significant additional defects in lung function, with severe mucus deposition within the airways, and consolidation of the alveolar spaces. These studies suggest that early-life respiratory viral infection leads to alterations in lung structure/repair that predispose to diminished lung function later in life.NEW & NOTEWORTHY These studies outline a novel finding that early-life respiratory virus infection can alter lung structure and function long-term. Importantly, the data also indicate that there are critical links between inflammatory responses and subsequent events that produce a more severe pathogenic response later in life. The findings provide additional data to support that early-life infections during lung development can alter the trajectory of airway function.
Keywords: RSV; lung function.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

