Phenotypic Spectrum of Progressive Supranuclear Palsy: Clinical Study and Apolipoprotein E Effect
- PMID: 38290492
- PMCID: PMC11082606
- DOI: 10.14802/jmd.23178
Phenotypic Spectrum of Progressive Supranuclear Palsy: Clinical Study and Apolipoprotein E Effect
Abstract
Objective: Progressive supranuclear palsy (PSP) is a rare neurodegenerative disorder encompassing several phenotypes with various motor and cognitive deficits. We aimed to study motor and cognitive characteristics across PSP phenotypes and to assess the influence of apolipoprotein E (APOE) gene variants on PSP phenotypic expression.
Methods: In this 20-year cross-sectional study, we retrospectively reviewed the charts of all patients classified as PSP patients and recategorized them according to phenotype using the Movement Disorder Society criteria (2017). Phenotypes were divided into three subgroups, Richardson's syndrome (PSP-RS), PSP-cortical (PSP with predominant frontal presentation [PSP-F] + PSP with predominant speech/language disorder [PSP-SL] + PSP with predominant corticobasal syndrome [PSP-CBS]) and PSP-subcortical (PSP with predominant parkinsonism [PSP-P] + PSP with progressive gait freezing [PSP-PGF] + PSP with predominant postural instability [PSP-PI] + PSP with predominant ocular motor dysfunction [PSP-OM] + PSP with cerebellar ataxia [PSP-C] + PSP with primary lateral sclerosis [PSP-PLS]), based on clinical presentation during the first 3 years after symptom onset, which defines the early disease stage. Clinical and neuropsychological assessment data were collected. Genotyping of APOE was performed using restriction fragment length polymorphism polymerase chain reaction and verified by Sanger sequencing.
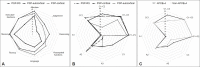
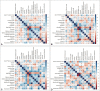
Results: We included 112 PSP patients comprising 10 phenotypes classified into 48 PSP-RS, 34 PSP-cortical (PSP-CBS, 17.6%; PSP-F, 9.4%; PSP-SL, 8.2%) and 30 PSP-subcortical (PSP-P, 11.6%; PSP-PI, 8%; PSP-OM, 2.7%; PSP-PGF, 1.8%; PSP-C, 1.8%; PSP-PLS, 0.9%) subgroups. PSP-RS patients were older at disease onset (p = 0.009) and had more akinetic-rigid and levodopa-resistant parkinsonism (p = 0.006), while PSP-cortical patients had more tremors and asymmetric and/or levodopa-responsive parkinsonism (p = 0.025). Cognitive domains were significantly less altered in the PSP-subcortical subgroup. Overall, PSP-APOEε4 carriers developed parkinsonism earlier (p = 0.038), had earlier oculomotor dysfunction (p = 0.052) and had more altered cognitive profiles. The APOEε4 allele was also associated with a younger age of parkinsonism onset in the PSP-RS phenotype group (p = 0.026).
Conclusion: This study demonstrated the wide phenotypic spectrum of PSP among Tunisians. Disease onset and akinetic-rigid and levodopa-resistant parkinsonism were the hallmarks of the PSP-RS phenotype, while milder cognitive impairment was characteristic of the PSP-subcortical subgroup. The APOEε4 allele was associated with earlier parkinsonism and oculomotor dysfunction and seemed to play a role in defining a more altered cognitive profile in PSP patients.
Keywords: Cognition; Cortical; Parkinsonism; Phenotypes; Progressive supranuclear palsy; Subcortical; Tauopathy.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

Similar articles
-
Association of PSP phenotypes with survival: A brain-bank study.Parkinsonism Relat Disord. 2021 Mar;84:77-81. doi: 10.1016/j.parkreldis.2021.01.015. Epub 2021 Feb 2. Parkinsonism Relat Disord. 2021. PMID: 33581485
-
Cognitive and behavioral profile of progressive supranuclear palsy and its phenotypes.J Neurol. 2021 Sep;268(9):3400-3408. doi: 10.1007/s00415-021-10511-y. Epub 2021 Mar 11. J Neurol. 2021. PMID: 33704556
-
Clinical, cognitive, and morphometric profiles of progressive supranuclear palsy phenotypes.J Neural Transm (Vienna). 2023 Feb;130(2):97-109. doi: 10.1007/s00702-023-02591-z. Epub 2023 Jan 26. J Neural Transm (Vienna). 2023. PMID: 36701008 Free PMC article.
-
Differential Diagnosis of Rare Subtypes of Progressive Supranuclear Palsy and PSP-Like Syndromes-Infrequent Manifestations of the Most Common Form of Atypical Parkinsonism.Front Aging Neurosci. 2022 Feb 9;14:804385. doi: 10.3389/fnagi.2022.804385. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35221993 Free PMC article. Review.
-
Progressive supranuclear palsy.Int Rev Neurobiol. 2019;149:49-86. doi: 10.1016/bs.irn.2019.10.013. Epub 2019 Nov 21. Int Rev Neurobiol. 2019. PMID: 31779824 Review.
Cited by
-
The Spectrum of Cognitive Impairment in Atypical Parkinsonism Syndromes: A Comprehensive Review of Current Understanding and Research.Diseases. 2025 Jan 31;13(2):39. doi: 10.3390/diseases13020039. Diseases. 2025. PMID: 39997046 Free PMC article. Review.
-
Comorbid pathologies and their impact on progressive supranuclear palsy: current view.J Neural Transm (Vienna). 2025 Aug 12. doi: 10.1007/s00702-025-03005-y. Online ahead of print. J Neural Transm (Vienna). 2025. PMID: 40794238 Review.
-
Pathomechanisms of neuropsychiatric disturbances in atypical parkinsonian disorders: a current view.J Neural Transm (Vienna). 2025 Apr;132(4):495-518. doi: 10.1007/s00702-025-02890-7. Epub 2025 Feb 15. J Neural Transm (Vienna). 2025. PMID: 39954076 Review.
-
Differences in neuropsychological performance across clinical variants of progressive supranuclear palsy.J Int Neuropsychol Soc. 2025 Aug 18:1-12. doi: 10.1017/S1355617725101215. Online ahead of print. J Int Neuropsychol Soc. 2025. PMID: 40820613 Free PMC article.
References
-
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. - PubMed
-
- Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23:394–400. - PubMed
-
- Stamelou M, Respondek G, Giagkou N, Whitwell JL, Kovacs GG, Höglinger GU. Evolving concepts in progressive supranuclear palsy and other 4-repeat tauopathies. Nat Rev Neurol. 2021;17:601–620. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

