Allogeneic hematopoietic cell transplantation is effective for p47phox chronic granulomatous disease: A Primary Immune Deficiency Treatment Consortium study
- PMID: 38290608
- PMCID: PMC11070290
- DOI: 10.1016/j.jaci.2024.01.013
Allogeneic hematopoietic cell transplantation is effective for p47phox chronic granulomatous disease: A Primary Immune Deficiency Treatment Consortium study
Abstract
Background: P47phox (neutrophil cytosolic factor-1) deficiency is the most common cause of autosomal recessive chronic granulomatous disease (CGD) and is considered to be associated with a milder clinical phenotype. Allogeneic hematopoietic cell transplantation (HCT) for p47phox CGD is not well-described.
Objectives: We sought to study HCT for p47phox CGD in North America.
Methods: Thirty patients with p47phox CGD who received allogeneic HCT at Primary Immune Deficiency Treatment Consortium centers since 1995 were included.
Results: Residual oxidative activity was present in 66.7% of patients. In the year before HCT, there were 0.38 CGD-related infections per person-years. Inflammatory diseases, predominantly of the lungs and bowel, occurred in 36.7% of the patients. The median age at HCT was 9.1 years (range 1.5-23.6 years). Most HCTs (90%) were performed after using reduced intensity/toxicity conditioning. HCT sources were HLA-matched (40%) and -mismatched (10%) related donors or HLA-matched (36.7%) and -mismatched (13.3%) unrelated donors. CGD-related infections after HCT decreased significantly to 0.06 per person-years (P = .038). The frequency of inflammatory bowel disease and the use of steroids also decreased. The cumulative incidence of graft failure and second HCT was 17.9%. The 2-year overall and event-free survival were 92.3% and 82.1%, respectively, while at 5 years they were 85.7% and 77.0%, respectively. In the surviving patients evaluated, ≥95% donor myeloid chimerism at 1 and 2 years after HCT was 93.8% and 87.5%, respectively.
Conclusions: Patients with p47phox CGD suffer from a significant disease burden that can be effectively alleviated by HCT. Similar to other forms of CGD, HCT should be considered for patients with p47phox CGD.
Keywords: Allogeneic hematopoietic cell transplantation; chronic granulomatous disease; p47phox.
Copyright © 2024 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest:
E. Grunebaum is an author for UpToDate and a consultant for Prime Medicine. R. A. Marsh is an author for UpToDate, an employee of Pharming Healthcare Inc and a consultant for Horizon Therapeutics. L. Murguía-Favela is on the Data Safety Monitoring Committee for Encoded Therapeutics. J. J. Bleesing is an author for UpToDate. E. L. Falcone has done advisory activities for Bruker Corporation. J. Heimall is an author for UpToDate, received an investigator-initiated grant from CSL Behring, and is a consultant for ADMA, CIRM, and Horizon. S. Prockop receives support for the conduct of sponsored trials from AlloVir and Jasper, and is a consultant for CellEvolve, Century, Pierre Fabre, and VOR Bio. S. Prockop received honoraria from Regeneron. B. J. Dávila Saldaña is on the Data Safety Monitoring Board for Orchard Therapeutics, and consultant for Sobi. M. J. Cowan is an author for UpToDate and is on the Data Safety Monitoring Board for Chiesi, Inc., Rocket Pharmaceuticals and Bluebird Bio. C. C. Dvorak is an author for UpToDate, is on the Data Safety Monitoring Board for Chiesi, and consultant for Orchard Therapeutics. E. Haddad is on the Data Safety Monitoring Board for Jasper and Rocket Pharma, and is a consultant for Prime Medicine, Takeda, and Octaphrama. O D. B. Kohn is an author for UpTo Date, is on the Data Safety Monitoring Board for Chiesi, and is a consultant/Scientific Advisory Board (SAB) member for ImmunoVec. J. M. Puck is an editor and author for UpToDate. M. A. Pulsipher reports study support from Adaptive and Miltenyi, working as an advisor for Vertex, Medexus, Equillium, Novartis, and Mesoblast, and receives educational honoraria from Novartis and Miltenyi. H. L. Malech serves without compensation as consultant/Scientific Advisory Board member for Jasper Therapeutics, Emendo Biotherapeutics, Orpha Labs, Ensoma, ImmunoVec, Curate Bio, and the DADA2 Foundation; as an advisor to and with Cooperative Research and Development Agreement support from CSL Behring and from Prime Medicine; and as Chair of an Independent Data Monitoring Committee for Rocket Pharmaceuticals. T. R. Torgerson is a consultant for Pharming Healthcare and is a member of the independent data safety monitoring board for Takeda. Other authors declare that they have no relevant conflicts of interest.
Figures
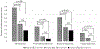
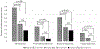
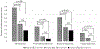


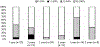
References
-
- Leiding JW, Holland SM. Chronic Granulomatous Disease. 2012 Aug 9 [updated 2022 Apr 21]. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 TR001263/TR/NCATS NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U54 NS064808/NS/NINDS NIH HHS/United States
- ZIA AI001222/ImNIH/Intramural NIH HHS/United States
- UG1 HL069254/HL/NHLBI NIH HHS/United States
- U10 HL069254/HL/NHLBI NIH HHS/United States
- R13 AI094943/AI/NIAID NIH HHS/United States
- U01 AI126612/AI/NIAID NIH HHS/United States
- U54 AI082973/AI/NIAID NIH HHS/United States
- ZIA AI000989/ImNIH/Intramural NIH HHS/United States
- Z01 AI000644/ImNIH/Intramural NIH HHS/United States
- U01 HL069294/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

