Association of a gene-expression subtype to outcome and treatment response in patients with recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab
- PMID: 38290766
- PMCID: PMC10828850
- DOI: 10.1136/jitc-2023-007823
Association of a gene-expression subtype to outcome and treatment response in patients with recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab
Abstract
Background: Immune checkpoint inhibitors have been approved and currently used in the clinical management of recurrent and metastatic head and neck squamous cell carcinoma (R/M HNSCC) patients. The reported benefit in clinical trials is variable and heterogeneous. Our study aims at exploring and comparing the predictive role of gene-expression signatures with classical biomarkers for immunotherapy-treated R/M HNSCC patients in a multicentric phase IIIb trial.
Methods: Clinical data were prospectively collected in Nivactor tiral (single-arm, open-label, multicenter, phase IIIb clinical trial in platinum-refractory HNSCC treated with nivolumab). Findings were validated in an external independent cohort of immune-treated HNSCC patients, divided in long-term and short-term survivors (overall survival >18 and <6 months since the start of immunotherapy, respectively). Pretreatment tumor tissue specimen from immunotherapy-treated R/M HNSCC patients was used for PD-L1 (Tumor Proportion Score; Combined Positive Score (CPS)) and Tumor Mutational Burden (Oncopanel TSO500) evaluation and gene expression profiling; classical biomarkers and immune signatures (retrieved from literature) were challenged in the NIVACTOR dataset.
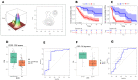
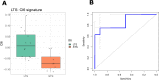
Results: Cluster-6 (Cl6) stratification of NIVACTOR cases in high score (n=16, 20%) and low score (n=64, 80%) demonstrated a statistically significant and clinically meaningful improvement in overall survival in the high-score cases (p=0.00028; HR=4.34, 95% CI 1.84 to 10.22) and discriminative ability reached area under the curve (AUC)=0.785 (95% CI 0.603 to 0.967). The association of high-score Cl6 with better outcome was also confirmed in: (1) NIVACTOR progression-free survival (p=4.93E-05; HR=3.71, 95% CI 1.92 to 7.18) and objective-response-rate (AUC=0.785; 95% CI 0.603 to 0.967); (2) long survivors versus short survivors (p=0.00544). In multivariate Cox regression analysis, Cl6 was independent from Eastern Cooperative Oncology Group performance status, PDL1-CPS, and primary tumor site.
Conclusions: These data highlight the presence of underlying biological differences able to predict survival and response following treatment with immunotherapy in platinum-refractory R/M HNSCC that could have translational implications improving treatment selection.
Trial registration number: EudraCT Number: 2017-000562-30.
Keywords: biomarkers, tumor; head and neck neoplasms; immunotherapy.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


References
-
- Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of Checkmate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. 10.1016/j.oraloncology.2018.04.008 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
