The MOG antibody non-P42 epitope is predictive of a relapsing course in MOG antibody-associated disease
- PMID: 38290838
- PMCID: PMC11103329
- DOI: 10.1136/jnnp-2023-332851
The MOG antibody non-P42 epitope is predictive of a relapsing course in MOG antibody-associated disease
Abstract
Background: Myelin oligodendrocyte glycoprotein (MOG) IgG seropositivity is a prerequisite for MOG antibody-associated disease (MOGAD) diagnosis. While a significant proportion of patients experience a relapsing disease, there is currently no biomarker predictive of disease course. We aim to determine whether MOG-IgG epitopes can predict a relapsing course in MOGAD patients.
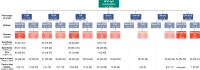
Methods: MOG-IgG-seropositive confirmed adult MOGAD patients were included (n=202). Serum MOG-IgG and epitope binding were determined by validated flow cytometry live cell-based assays. Associations between epitopes, disease course, clinical phenotype, Expanded Disability Status Scale and Visual Functional System Score at onset and last review were evaluated.
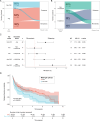
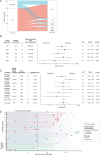
Results: Of 202 MOGAD patients, 150 (74%) patients had MOG-IgG that recognised the immunodominant proline42 (P42) epitope and 115 (57%) recognised histidine103/serine104 (H103/S104). Fifty-two (26%) patients had non-P42 MOG-IgG and showed an increased risk of a relapsing course (HR 1.7; 95% CI 1.15 to 2.60, p=0.009). Relapse-freedom was shorter in patients with non-P42 MOG-IgG (p=0.0079). Non-P42 MOG-IgG epitope status remained unchanged from onset throughout the disease course and was a strong predictor of a relapsing course in patients with unilateral optic neuritis (HR 2.7, 95% CI 1.06 to 6.98, p=0.038), with high specificity (95%, 95% CI 77% to 100%) and positive predictive value (85%, 95% CI 45% to 98%).
Conclusions: Non-P42 MOG-IgG predicts a relapsing course in a significant subgroup of MOGAD patients. Patients with unilateral optic neuritis, the most frequent MOGAD phenotype, can reliably be tested at onset, regardless of age and sex. Early detection and specialised management in these patients could minimise disability and improve long-term outcomes.
Keywords: IMMUNOLOGY; MULTIPLE SCLEROSIS; NEUROIMMUNOLOGY.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MF-P has received research grant from MS Australia and travel compensation from Merck. MB reports research grants from Genzyme-Sanofi, Novartis, Biogen, Merck and BMS; and is a Research Consultant for RxMx and Research Director for the Sydney Neuroimaging Analysis Centre. SWR has received funds over the last 5 years including but not limited to travel support, honoraria, trial payments, research and clinical support to the neurology department or academic projects of which he is a member has been received from bodies and charities: NHMRC, NBA, MAA, Lambert Initiative, Beeren foundation, anonymous donors; and from pharmaceutical/biological companies: Alexion, Biogen, CSL, Genzyme, Grifols, Merck, Novartis, Roche, Sanofi. MM has served on scientific and commercial advisory boards for Merck Serono. AvdW has received travel support from Merck Serono, Novartis, Biogen, Roche and Sanofi. She has served on scientific and commercial advisory boards for Merck, Novartis, Sanofi and Roche, and has received unencumbered research grants from Novartis, Biogen, Merck and Roche. JLS has accepted travel compensation from Novartis, Biogen and Merck Serono. Her institution receives the honoraria for talks and advisory board commitment as well as research grants from Bayer Health Care, Biogen, Genzyme Sanofi, Merck, Novartis and TEVA. AGK has in recent times received speaker honoraria and Scientific Advisory Board fees from Bayer, BioCSL, Biogen-Idec, Lgpharma, Merck, Novartis, Roche, Sanofi-Aventis, Sanofi-Genzyme, Teva, NeuroScientific Biopharmaceuticals, Innate Immunotherapeutics, and Mitsubishi Tanabe Pharma. His work has received grant funding from the Eyewall Foundation, Trish MS Foundation, MS Australia, MS Western Australia, the MS Base Foundation, the National Health and Medical Research Council of Australia, and the National Multiple Sclerosis Society, USA. He is an investigator in clinical trials sponsored by Biogen Idec and Novartis. TK served on scientific advisory boards for BMS, Roche, Janssen, Sanofi Genzyme, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Sanofi Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Sanofi-Genzyme, Teva, BioCSL and Merck and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck. SAB has received honoraria for attendance at advisory boards and travel sponsorship from Bayer-Schering, Biogen-Idec, Merck-Serono, Novartis and Sanofi-Genzyme, has received speakers honoraria from Biogen-Idec and Genzyme, is an investigator in clinical trials sponsored by Biogen Idec, Novartis and Genzyme, and was the recipient of an unencumbered research grant from Biogen-Idec. RCD received research funding from the Petre Foundation and National Health and Medical Research Council (Australia). He has received honoraria form Biogen Idec and Merck Serono as invited speaker. SR received research funding from the National Health and Medical Research Council (Australia), the Royal Australasian College of Physicians, and the University of Sydney. She serves as a consultant on an advisory board for UCB and Limbic Neurology, The MOG Project and the Sumaira Foundation, and has been an invited speaker for Biogen, Excemed, Alexxion, Limbic Neurology, and Novartis. FB has received research funding from NSW Health, MS Australia, the National Health Medical Research Council (Australia), the Medical Research Future Fund (Australia) and investigator-initiated research grant from Novartis. She was on an advisory boards for Novartis, Merck and The MOG Project and the Sumaira Foundation, and has been an invited speaker for Biogen, Novartis and Limbic Neurology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
