Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications
- PMID: 38291025
- PMCID: PMC10828500
- DOI: 10.1038/s41467-024-44701-9
Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications
Abstract
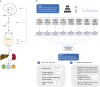
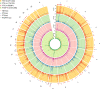
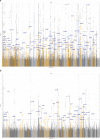
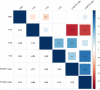
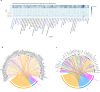
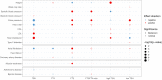
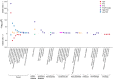
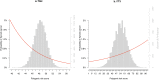
To date only a fraction of the genetic footprint of thyroid function has been clarified. We report a genome-wide association study meta-analysis of thyroid function in up to 271,040 individuals of European ancestry, including reference range thyrotropin (TSH), free thyroxine (FT4), free and total triiodothyronine (T3), proxies for metabolism (T3/FT4 ratio) as well as dichotomized high and low TSH levels. We revealed 259 independent significant associations for TSH (61% novel), 85 for FT4 (67% novel), and 62 novel signals for the T3 related traits. The loci explained 14.1%, 6.0%, 9.5% and 1.1% of the total variation in TSH, FT4, total T3 and free T3 concentrations, respectively. Genetic correlations indicate that TSH associated loci reflect the thyroid function determined by free T3, whereas the FT4 associations represent the thyroid hormone metabolism. Polygenic risk score and Mendelian randomization analyses showed the effects of genetically determined variation in thyroid function on various clinical outcomes, including cardiovascular risk factors and diseases, autoimmune diseases, and cancer. In conclusion, our results improve the understanding of thyroid hormone physiology and highlight the pleiotropic effects of thyroid function on various diseases.
© 2024. The Author(s).
Conflict of interest statement
B.M.P. serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson not directly related to this project. The remaining authors declare no competing interests.
Figures
References
-
- Maenhaut, C. et al. Ontogeny, anatomy, metabolism and physiology of the thyroid. in Endotext (eds. Feingold, K. R. et al.) (South Dartmouth (MA), 2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

