Comparison of cytotoxicity of Miltefosine and its niosomal form on chick embryo model
- PMID: 38291076
- PMCID: PMC10827708
- DOI: 10.1038/s41598-024-52620-4
Comparison of cytotoxicity of Miltefosine and its niosomal form on chick embryo model
Abstract
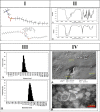
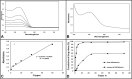
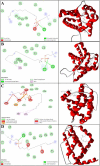
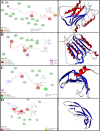
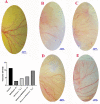
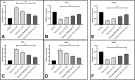
Various drugs have been used for the treatment of leishmaniasis, but they often have adverse effects on the body's organs. In this study, we aimed to explore the effects of one type of drug, Miltefosine (MIL), and its analogue or modifier, liposomal Miltefosine (NMIL), on several fetal organs using both in silico analysis and practical tests on chicken embryos. Our in silico approach involved predicting the affinities of MIL and NMIL to critical proteins involved in leishmaniasis, including Vascular Endothelial Growth Factor A (VEGF-A), the Kinase insert domain receptor (KDR1), and apoptotic-regulator proteins (Bcl-2-associate). We then validated and supported these predictions through in vivo investigations, analyzing gene expression and pathological changes in angiogenesis and apoptotic mediators in MIL- and NMIL-treated chicken embryos. The results showed that NMIL had a more effective action towards VEGF-A and KDR1 in leishmaniasis, making it a better candidate for potential operative treatment during pregnancy than MIL alone. In vivo, studies also showed that chicken embryos under MIL treatment displayed less vascular mass and more degenerative and apoptotic changes than those treated with NMIL. These results suggest that NMIL could be a better treatment option for leishmaniasis during pregnancy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis.PLoS Negl Trop Dis. 2012;6(5):e1657. doi: 10.1371/journal.pntd.0001657. Epub 2012 May 22. PLoS Negl Trop Dis. 2012. PMID: 22629478 Free PMC article.
-
Cytotoxicity of Amphotericin B and AmBisome: In Silico and In Vivo Evaluation Employing the Chick Embryo Model.Front Pharmacol. 2022 Jun 8;13:860598. doi: 10.3389/fphar.2022.860598. eCollection 2022. Front Pharmacol. 2022. PMID: 35754489 Free PMC article.
-
Assessing the Efficacy and Safety of Liposomal Amphotericin B and Miltefosine in Combination for Treatment of Post Kala-Azar Dermal Leishmaniasis.J Infect Dis. 2020 Feb 3;221(4):608-617. doi: 10.1093/infdis/jiz486. J Infect Dis. 2020. PMID: 31854451
-
Miltefosine: an oral drug for visceral leishmaniasis.Indian J Pediatr. 2004 Feb;71(2):143-4. doi: 10.1007/BF02723096. Indian J Pediatr. 2004. PMID: 15053378 Review.
-
[Miltefosine: a new remedy for leishmaniasis].Ned Tijdschr Geneeskd. 2006 Dec 9;150(49):2697-701. Ned Tijdschr Geneeskd. 2006. PMID: 17194005 Review. Dutch.
Cited by
-
The synergistic anti-leishmanial effect of photodynamic therapy employing chemotherapy-mediated nanocomposites.Sci Rep. 2025 May 10;15(1):16282. doi: 10.1038/s41598-025-01097-w. Sci Rep. 2025. PMID: 40348806 Free PMC article.
-
Discovery of Cyclopentane-Based Phospholipids as Miltefosine Analogs with Superior Potency and Enhanced Selectivity Against Naegleria fowleri.Pharmaceuticals (Basel). 2025 Jun 30;18(7):984. doi: 10.3390/ph18070984. Pharmaceuticals (Basel). 2025. PMID: 40732274 Free PMC article.
-
Exploring the potential of silymarin-loaded nanovesicles as an effective drug delivery system for cancer therapy: in vivo, in vitro, and in silico experiments.Naunyn Schmiedebergs Arch Pharmacol. 2024 Sep;397(9):7017-7036. doi: 10.1007/s00210-024-03099-3. Epub 2024 Apr 17. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38630254
References
-
- Bahraminegad S, Pardakhty A, Sharifi I, Ranjbar M. Therapeutic effects of the as-synthesized polylactic acid/chitosan nanofibers decorated with amphotricin B for in vitro treatment of leishmaniasis. J. Saudi Chem. Soc. 2021;25:101362. doi: 10.1016/j.jscs.2021.101362. - DOI
-
- Roatt, B. M. et al. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol., 1–13 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

