LOGGIC/FIREFLY-2: a phase 3, randomized trial of tovorafenib vs. chemotherapy in pediatric and young adult patients with newly diagnosed low-grade glioma harboring an activating RAF alteration
- PMID: 38291372
- PMCID: PMC10826080
- DOI: 10.1186/s12885-024-11820-x
LOGGIC/FIREFLY-2: a phase 3, randomized trial of tovorafenib vs. chemotherapy in pediatric and young adult patients with newly diagnosed low-grade glioma harboring an activating RAF alteration
Abstract
Background: Pediatric low-grade glioma (pLGG) is essentially a single pathway disease, with most tumors driven by genomic alterations affecting the mitogen-activated protein kinase/ERK (MAPK) pathway, predominantly KIAA1549::BRAF fusions and BRAF V600E mutations. This makes pLGG an ideal candidate for MAPK pathway-targeted treatments. The type I BRAF inhibitor, dabrafenib, in combination with the MEK inhibitor, trametinib, has been approved by the United States Food and Drug Administration for the systemic treatment of BRAF V600E-mutated pLGG. However, this combination is not approved for the treatment of patients with tumors harboring BRAF fusions as type I RAF inhibitors are ineffective in this setting and may paradoxically enhance tumor growth. The type II RAF inhibitor, tovorafenib (formerly DAY101, TAK-580, MLN2480), has shown promising activity and good tolerability in patients with BRAF-altered pLGG in the phase 2 FIREFLY-1 study, with an objective response rate (ORR) per Response Assessment in Neuro-Oncology high-grade glioma (RANO-HGG) criteria of 67%. Tumor response was independent of histologic subtype, BRAF alteration type (fusion vs. mutation), number of prior lines of therapy, and prior MAPK-pathway inhibitor use.
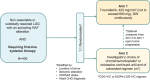
Methods: LOGGIC/FIREFLY-2 is a two-arm, randomized, open-label, multicenter, global, phase 3 trial to evaluate the efficacy, safety, and tolerability of tovorafenib monotherapy vs. current standard of care (SoC) chemotherapy in patients < 25 years of age with pLGG harboring an activating RAF alteration who require first-line systemic therapy. Patients are randomized 1:1 to either tovorafenib, administered once weekly at 420 mg/m2 (not to exceed 600 mg), or investigator's choice of prespecified SoC chemotherapy regimens. The primary objective is to compare ORR between the two treatment arms, as assessed by independent review per RANO-LGG criteria. Secondary objectives include comparisons of progression-free survival, duration of response, safety, neurologic function, and clinical benefit rate.
Discussion: The promising tovorafenib activity data, CNS-penetration properties, strong scientific rationale combined with the manageable tolerability and safety profile seen in patients with pLGG led to the SIOPe-BTG-LGG working group to nominate tovorafenib for comparison with SoC chemotherapy in this first-line phase 3 trial. The efficacy, safety, and functional response data generated from the trial may define a new SoC treatment for newly diagnosed pLGG.
Trial registration: ClinicalTrials.gov: NCT05566795. Registered on October 4, 2022.
Keywords: BRAF; Chemotherapy; Child; First-line; MAPK; Pediatric low-grade glioma; Tovorafenib; pLGG.
© 2024. The Author(s).
Conflict of interest statement
The authors wish to declare the following competing interests: C.M.vT has participated in advisory boards for Novartis, Bayer, and Alexion. L.B.K has received consulting fees from Blueprint Medicine and contracted research from Novartis, Regeneron Pharmaceuticals, Day One Biopharmaceuticals, Spring Works Therapeutics, Bristol Myers Squibb, and SonALAsense and also is a stock shareholder at Onconova Therapeutics. S.P has participated in advisory boards for Bayer, Alexion, and Esai and received research support from Novartis, Bayer, and Roche. A.A.A has participated in an advisory board for Alexion and received travel and scientific grants from Alexion. M.Z has participated in an advisory board for AstraZeneca. K.S has participated in advisory boards for Alexion, Servier, Jazz Pharmaceuticals, Novartis, Roche, Bayer, and NovoNordisk. A.S has participated in advisory boards for Alexion, AstraZeneca Rare Disease, and Day One Biopharmaceuticals. E.O has participated in an advisory board for Alexion/AstraZeneca Rare Disease. P.HD has participated in an advisory board and consultancy for Alexion. D.S.Z has received consulting fees from Bayer, AstraZeneca, Accendatech, Novartis, Day One Biopharmaceuticals, FivePhusion, Amgen, Alexion, and Norgine and also received research support from Accendatech. D.C is a co-founder and shareholder for Heidelberg Epignostix GmbH and receives royalties for sale of BRAF V600E specific antibody VE1. F.S has received honoraria from Bayer and Illumina and is a co-founder and shareholder for Heidelberg Epignostix GmbH. J.Q, L-P.T, S.C.B, and P.M are all employees of Day One Biopharmaceuticals and have received Day One Biopharmaceuticals stock and stock options. T.M has received research grants from Day One Biopharmaceuticals and Biomed Valley Discovery. D.T.W.J is a co-founder and shareholder for Heidelberg Epignostix GmbH. D.H has participated in advisory boards for Alexion/AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Day One Biopharmaceuticals, Janssen, Novartis, and Roche and also received research grants from Alexion/AstraZeneca and Roche. O.W has participated in advisory boards for Novartis, Janssen, Roche, Bristol Myers Squibb, and AstraZeneca and also received research grants from Day One Biopharmaceuticals, Biomed Valley Discovery, Bristol Myers Squibb, Syndax, and PreComb. The following authors have no competing interests to declare: R.S, O.C, A. Y. N. SvM, S.A, A.K, and R.W.
Figures
References
-
- Ostrom QT, Price M, Ryan K, Edelson J, Neff C, Cioffi G, et al. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2022;24(Suppl 3):iii1–38. doi: 10.1093/neuonc/noac161. - DOI - PMC - PubMed
-
- Gnekow AK, Falkenstein F, von Hornstein S, Zwiener I, Berkefeld S, Bison B, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. doi: 10.1093/neuonc/nos202. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

