New insights into the role of mitochondrial metabolic dysregulation and immune infiltration in septic cardiomyopathy by integrated bioinformatics analysis and experimental validation
- PMID: 38291374
- PMCID: PMC10826082
- DOI: 10.1186/s11658-024-00536-2
New insights into the role of mitochondrial metabolic dysregulation and immune infiltration in septic cardiomyopathy by integrated bioinformatics analysis and experimental validation
Abstract
Background: Septic cardiomyopathy (SCM), a common cardiovascular comorbidity of sepsis, has emerged among the leading causes of death in patients with sepsis. SCM's pathogenesis is strongly affected by mitochondrial metabolic dysregulation and immune infiltration disorder. However, the specific mechanisms and their intricate interactions in SCM remain unclear. This study employed bioinformatics analysis and drug discovery approaches to identify the regulatory molecules, distinct functions, and underlying interactions of mitochondrial metabolism and immune microenvironment, along with potential interventional strategies in SCM.
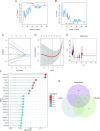
Methods: GSE79962, GSE171546, and GSE167363 datasets were obtained from the Gene Expression Omnibus (GEO) database. Differentially expressed genes (DEGs) and module genes were identified using Limma and Weighted Correlation Network Analysis (WGCNA), followed by functional enrichment analysis. Machine learning algorithms, including support vector machine-recursive feature elimination (SVM-RFE), least absolute shrinkage and selection operator (LASSO) regression, and random forest, were used to screen mitochondria-related hub genes for early diagnosis of SCM. Subsequently, a nomogram was developed based on six hub genes. The immunological landscape was evaluated by single-sample gene set enrichment analysis (ssGSEA). We also explored the expression pattern of hub genes and distribution of mitochondria/inflammation-related pathways in UMAP plots of single-cell dataset. Potential drugs were explored using the Drug Signatures Database (DSigDB). In vivo and in vitro experiments were performed to validate the pathogenetic mechanism of SCM and the therapeutic efficacy of candidate drugs.
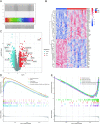
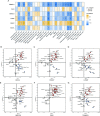
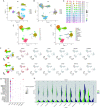
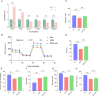
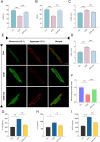
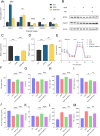
Results: Six hub mitochondria-related DEGs [MitoDEGs; translocase of inner mitochondrial membrane domain-containing 1 (TIMMDC1), mitochondrial ribosomal protein S31 (MRPS31), F-box only protein 7 (FBXO7), phosphatidylglycerophosphate synthase 1 (PGS1), LYR motif containing 7 (LYRM7), and mitochondrial chaperone BCS1 (BCS1L)] were identified. The diagnostic nomogram model based on the six hub genes demonstrated high reliability and validity in both the training and validation sets. The immunological microenvironment differed between SCM and control groups. The Spearman correlation analysis revealed that hub MitoDEGs were significantly associated with the infiltration of immune cells. Upregulated hub genes showed remarkably high expression in the naive/memory B cell, CD14+ monocyte, and plasma cell subgroup, evidenced by the feature plot. The distribution of mitochondria/inflammation-related pathways varied across subgroups among control and SCM individuals. Metformin was predicted to be the most promising drug with the highest combined score. Its efficacy in restoring mitochondrial function and suppressing inflammatory responses has also been validated.
Conclusions: This study presents a comprehensive mitochondrial metabolism and immune infiltration landscape in SCM, providing a potential novel direction for the pathogenesis and medical intervention of SCM.
Keywords: Drug discovery; Immune infiltration; Mitochondrial metabolism; Molecular mechanism; Septic cardiomyopathy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no potential competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

