Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression
- PMID: 38291438
- PMCID: PMC10829273
- DOI: 10.1186/s13046-024-02943-x
Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression
Abstract
Background: Discoidin, CUB, and LCCL domain-containing type I (DCBLD1) is identified as an oncogene involved in multiple regulation of tumor progression, but specific mechanisms remain unclear in cervical cancer. Lactate-mediated lactylation modulates protein function. Whether DCBLD1 can be modified by lactylation and the function of DCBLD1 lactylation are unknown. Therefore, this study aims to investigate the lactylation of DCBLD1 and identify its specific lactylation sites. Herein, we elucidated the mechanism by which lactylation modification stabilizes the DCBLD1 protein. Furthermore, we investigated DCBLD1 overexpression activating pentose phosphate pathway (PPP) to promote the progression of cervical cancer.
Methods: DCBLD1 expression was examined in human cervical cancer cells and adjacent non-tumorous tissues using quantitative reverse transcription-polymerase chain reaction, western blotting, and immunohistochemistry. In vitro and in vivo studies were conducted to investigate the impact of DCBLD1 on the progression of cervical cancer. Untargeted liquid chromatography-tandem mass spectrometry (LC-MS/MS) metabolomics studies were used to characterize DCBLD1-induced metabolite alterations. Western blot, immunofuorescence and transmission electron microscopy were performed to detect DCBLD1 degradation of G6PD by activating autophagy. Chromatin immunoprecipitation, dual luciferase reporter assay for detecting the mechanism by which lactate increases DCBLD1 transcription. LC-MS/MS was employed to verify specific modification sites within the DCBLD1 protein.
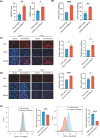
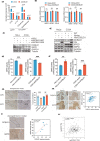
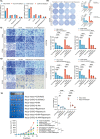
Results: We found that lactate increased DCBLD1 expression, activating the PPP to facilitate the proliferation and metastasis of cervical cancer cells. DCBLD1 primarily stimulated PPP by upregulating glucose-6-phosphate dehydrogenase (G6PD) expression and enzyme activity. The mechanism involved the increased enrichment of HIF-1α in the DCBLD1 promoter region, enhancing the DCBLD1 mRNA expression. Additionally, lactate-induced DCBLD1 lactylation stabilized DCBLD1 expression. We identified DCBLD1 as a lactylation substrate, with a predominant lactylation site at K172. DCBLD1 overexpression inhibited G6PD autophagic degradation, activating PPP to promote cervical cancer progression. In vivo, 6-An mediated inhibition of G6PD enzyme activity, inhibiting tumor proliferation.
Conclusions: Our findings revealed a novel post-translational modification type of DCBDL1, emphasizing the significance of lactylation-driven DCBDL1-mediated PPP in promoting the progression of cervical cancer.
Keywords: Autophagy; Cervical cancer; DCBLD1; G6PD; HIF-1α; Lactylation; Pentose phosphate pathway.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

