The evolving roles of Wnt signaling in stem cell proliferation and differentiation, the development of human diseases, and therapeutic opportunities
- PMID: 38292186
- PMCID: PMC10825312
- DOI: 10.1016/j.gendis.2023.04.042
The evolving roles of Wnt signaling in stem cell proliferation and differentiation, the development of human diseases, and therapeutic opportunities
Abstract
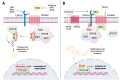
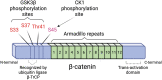
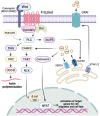
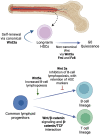
The evolutionarily conserved Wnt signaling pathway plays a central role in development and adult tissue homeostasis across species. Wnt proteins are secreted, lipid-modified signaling molecules that activate the canonical (β-catenin dependent) and non-canonical (β-catenin independent) Wnt signaling pathways. Cellular behaviors such as proliferation, differentiation, maturation, and proper body-axis specification are carried out by the canonical pathway, which is the best characterized of the known Wnt signaling paths. Wnt signaling has emerged as an important factor in stem cell biology and is known to affect the self-renewal of stem cells in various tissues. This includes but is not limited to embryonic, hematopoietic, mesenchymal, gut, neural, and epidermal stem cells. Wnt signaling has also been implicated in tumor cells that exhibit stem cell-like properties. Wnt signaling is crucial for bone formation and presents a potential target for the development of therapeutics for bone disorders. Not surprisingly, aberrant Wnt signaling is also associated with a wide variety of diseases, including cancer. Mutations of Wnt pathway members in cancer can lead to unchecked cell proliferation, epithelial-mesenchymal transition, and metastasis. Altogether, advances in the understanding of dysregulated Wnt signaling in disease have paved the way for the development of novel therapeutics that target components of the Wnt pathway. Beginning with a brief overview of the mechanisms of canonical and non-canonical Wnt, this review aims to summarize the current knowledge of Wnt signaling in stem cells, aberrations to the Wnt pathway associated with diseases, and novel therapeutics targeting the Wnt pathway in preclinical and clinical studies.
Keywords: Cancer; Canonical Wnt; Disease; Non-canonical Wnt; Stem cells; Targeted therapy; Wnt signaling; β-Catenin.
© 2023 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Figures



References
-
- Nusse R., Varmus H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. - PubMed
-
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50(4):649–657. - PubMed
-
- Wodarz A., Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. - PubMed
-
- Hayat R., Manzoor M., Hussain A. Wnt signaling pathway: a comprehensive review. Cell Biol Int. 2022;46(6):863–877. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

