Torsional flexibility in zinc-benzenedicarboxylate metal-organic frameworks
- PMID: 38293003
- PMCID: PMC10823780
- DOI: 10.1039/d3ce01078c
Torsional flexibility in zinc-benzenedicarboxylate metal-organic frameworks
Abstract
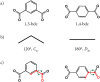
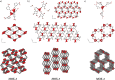
We explore the role and nature of torsional flexibility of carboxylate-benzene links in the structural chemistry of metal-organic frameworks (MOFs) based on Zn and benzenedicarboxlyate (bdc) linkers. A particular motivation is to understand the extent to which such flexibility is important in stabilising the unusual topologically aperiodic phase known as TRUMOF-1. We compare the torsion angle distributions of TRUMOF-1 models with those for crystalline Zn/1,3-bdc MOFs, including a number of new materials whose structures we report here. We find that both periodic and aperiodic Zn/1,3-bdc MOFs sample a similar range of torsion angles, and hence the formation of TRUMOF-1 does not require any additional flexibility beyond that already evident in chemically-related crystalline phases. Comparison with Zn/1,4-bdc MOFs does show, however, that the lower symmetry of the 1,3-bdc linker allows access to a broader range of torsion angles, reflecting a greater flexibility of this linker.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Lee J. Y. Olson D. H. Pan L. Emge T. J. Li J. Adv. Funct. Mater. 2007;17:1255–1262. doi: 10.1002/adfm.200600944. - DOI
-
- Kumar P. Paul A. K. Deep A. Microporous Mesoporous Mater. 2014;195:60–66. doi: 10.1016/j.micromeso.2014.04.017. - DOI
-
- Chen M.-L. Qi Z.-L. Jin W.-T. Xu Z. Cheng Y.-H. Zhou Z.-H. CrystEngComm. 2021;23:7442–7449. doi: 10.1039/D1CE00931A. - DOI
LinkOut - more resources
Full Text Sources
