This is a preprint.
Development and characterization of phospho-ubiquitin antibodies to monitor PINK1-PRKN signaling in cells and tissue
- PMID: 38293125
- PMCID: PMC10827112
- DOI: 10.1101/2024.01.15.575715
Development and characterization of phospho-ubiquitin antibodies to monitor PINK1-PRKN signaling in cells and tissue
Update in
-
Development and characterization of phospho-ubiquitin antibodies to monitor PINK1-PRKN signaling in cells and tissue.Autophagy. 2024 Sep;20(9):2076-2091. doi: 10.1080/15548627.2024.2356490. Epub 2024 May 27. Autophagy. 2024. PMID: 38802071 Free PMC article.
Abstract
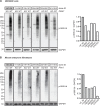
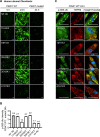
The selective removal of dysfunctional mitochondria, a process termed mitophagy, is critical for cellular health and impairments have been linked to aging, Parkinson disease, and other neurodegenerative conditions. A central mitophagy pathway is orchestrated by the ubiquitin (Ub) kinase PINK1 together with the E3 Ub ligase PRKN/Parkin. The decoration of damaged mitochondrial domains with phosphorylated Ub (p-S65-Ub) mediates their elimination though the autophagy system. As such p-S65-Ub has emerged as a highly specific and quantitative marker of mitochondrial damage with significant disease relevance. Existing p-S65-Ub antibodies have been successfully employed as research tools in a range of applications including western blot, immunocytochemistry, immunohistochemistry, and ELISA. However, physiological levels of p-S65-Ub in the absence of exogenous stress are very low, therefore difficult to detect and require reliable and ultrasensitive methods. Here we generated and characterized a collection of novel recombinant, rabbit monoclonal p-S65-Ub antibodies with high specificity and affinity in certain applications that allow the field to better understand the molecular mechanisms and disease relevance of PINK1-PRKN signaling. These antibodies may also serve as novel diagnostic or prognostic tools to monitor mitochondrial damage in various clinical and pathological specimens.
Keywords: PINK1; PRKN; Parkin; Parkinson disease; autophagy; mitochondria; mitophagy; phospho-ubiquitin; recombinant antibody; ubiquitin.
Conflict of interest statement
DISCLOSURE STATEMENT Mayo Clinic, F.C.F., and W.S. have filed a patent related to PRKN activators. Additional funding sources to disclose but not pertinent to the current study include a grant from Amazentis SA (to W.S.). Z.K.W. serves as PI or Co-PI on projects/grants from Biohaven Pharmaceuticals, Inc. and Vigil Neuroscience, Inc., and is an external advisory board member for Vigil Neuroscience, Inc., and a consultant on neurodegenerative medical research for Eli Lilly & Company. J.B.F. was CEO at 21st Century Biochemicals Inc., K.P. is an employee of ImmunoPrecise Antibodies Ltd., and R.K. is an employee of Abcam Plc.. All other authors declare they have no competing interests. This research was conducted in compliance with Mayo Clinic conflict of interest policies.
Figures





References
-
- Tanaka K. The PINK1-Parkin axis: An Overview. Neurosci Res. 2020. Oct;159:9–15. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
