This is a preprint.
MAIT Cells Modulate Innate Immune Cells and Inhibit Colon Cancer Growth
- PMID: 38293128
- PMCID: PMC10827136
- DOI: 10.1101/2024.01.16.575894
MAIT Cells Modulate Innate Immune Cells and Inhibit Colon Cancer Growth
Update in
-
Mucosal-associated invariant T cells modulate innate immune cells and inhibit colon cancer growth.Scand J Immunol. 2024 Sep;100(3):e13391. doi: 10.1111/sji.13391. Epub 2024 May 21. Scand J Immunol. 2024. PMID: 38773691 Free PMC article.
Abstract
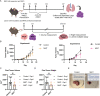
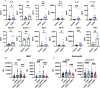
Mucosal-associated invariant T (MAIT) cells are innate-like T cells that can be activated by microbial antigens and cytokines and are abundant in mucosal tissues including the colon. MAIT cells have cytotoxic and pro-inflammatory functions and have potentials for use as adoptive cell therapy. However, studies into their anti-cancer activity, including their role in colon cancer, are limited. Using an animal model of colon cancer, we show that peritumoral injection of in vivo-expanded MAIT cells into RAG1-/- mice with MC38-derived tumors inhibits tumor growth compared to control. Multiplex cytokine analyses show that tumors from the MAIT cell-treated group have higher expression of markers for eosinophil-activating cytokines, suggesting an association between eosinophil recruitment and tumor inhibition. In a human peripheral leukocyte co-culture model, we show that leukocytes stimulated with MAIT ligand show an increase in eotaxin-1 production and activation of eosinophils, associated with increased cancer cell killing. In conclusion, we show that MAIT cells have a protective role in a murine colon cancer model, associated with modulation of the immune response to cancer, potentially involving eosinophil-associated mechanisms. Our results highlight the potential of MAIT cells for non-donor restricted colon cancer immunotherapy.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
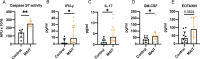

References
-
- Treiner E, Duban L, Bahram S, Radosavljevic R, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422. - PubMed
-
- Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–8. - PubMed
-
- Hagel J, Garner L, Bilton M, Mehta H, Leng T, Hackstein C, Phalora P, Amini A, Akther H, Provine N, Edmans M, Willberg C, Klenerman P. Human MAIT Cell Activation. Methods in Moelcular Biology. 2020;2098:97–124. - PubMed
-
- Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, Mettke E, Kurioka A, Hansen TH, Klenerman P, Willberg CB. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
