This is a preprint.
NRV: An open framework for in silico evaluation of peripheral nerve electrical stimulation strategies
- PMID: 38293181
- PMCID: PMC10827078
- DOI: 10.1101/2024.01.15.575628
NRV: An open framework for in silico evaluation of peripheral nerve electrical stimulation strategies
Update in
-
NRV: An open framework for in silico evaluation of peripheral nerve electrical stimulation strategies.PLoS Comput Biol. 2024 Jul 12;20(7):e1011826. doi: 10.1371/journal.pcbi.1011826. eCollection 2024 Jul. PLoS Comput Biol. 2024. PMID: 38995970 Free PMC article.
Abstract
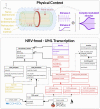
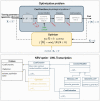
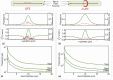
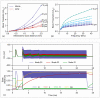
Electrical stimulation of peripheral nerves has been used in various pathological contexts for rehabilitation purposes or to alleviate the symptoms of neuropathologies, thus improving the overall quality of life of patients. However, the development of novel therapeutic strategies is still a challenging issue requiring extensive in vivo experimental campaigns and technical development. To facilitate the design of new stimulation strategies, we provide a fully open source and self-contained software framework for the in silico evaluation of peripheral nerve electrical stimulation. Our modeling approach, developed in the popular and well-established Python language, uses an object-oriented paradigm to map the physiological and electrical context. The framework is designed to facilitate multi-scale analysis, from single fiber stimulation to whole multifascicular nerves. It also allows the simulation of complex strategies such as multiple electrode combinations and waveforms ranging from conventional biphasic pulses to more complex modulated kHz stimuli. In addition, we provide automated support for stimulation strategy optimization and handle the computational backend transparently to the user. Our framework has been extensively tested and validated with several existing results in the literature.
Figures




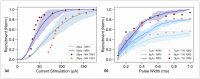


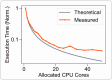
Similar articles
-
NRV: An open framework for in silico evaluation of peripheral nerve electrical stimulation strategies.PLoS Comput Biol. 2024 Jul 12;20(7):e1011826. doi: 10.1371/journal.pcbi.1011826. eCollection 2024 Jul. PLoS Comput Biol. 2024. PMID: 38995970 Free PMC article.
-
Modulating individual axons and axonal populations in the peripheral nerve using transverse intrafascicular multichannel electrodes.J Neural Eng. 2023 Aug 22;20(4). doi: 10.1088/1741-2552/aced20. J Neural Eng. 2023. PMID: 37536318
-
Accurate simulation of cuff electrode stimulation predicting in-vivo strength-duration thresholds.Artif Organs. 2022 Oct;46(10):2073-2084. doi: 10.1111/aor.14374. Epub 2022 Aug 9. Artif Organs. 2022. PMID: 35896504 Free PMC article.
-
A Multiscale Approach to Axon and Nerve Stimulation Modeling: A Review.IEEE Trans Neural Syst Rehabil Eng. 2021;29:397-407. doi: 10.1109/TNSRE.2021.3054551. Epub 2021 Mar 2. IEEE Trans Neural Syst Rehabil Eng. 2021. PMID: 33497336 Review.
-
Validation of a parameterized, open-source model of nerve stimulation.J Neural Eng. 2021 Aug 11;18(4):10.1088/1741-2552/ac1983. doi: 10.1088/1741-2552/ac1983. J Neural Eng. 2021. PMID: 34330105 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
