Multiplex solid-phase RPA coupled CRISPR-based visual detection of SARS-CoV-2
- PMID: 38293281
- PMCID: PMC10827331
- DOI: 10.1016/j.biosx.2023.100381
Multiplex solid-phase RPA coupled CRISPR-based visual detection of SARS-CoV-2
Abstract
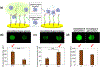
The COVID-19 pandemic has presented a significant challenge to the world's public health and led to over 6.9 million deaths reported to date. A rapid, sensitive, and cost-effective point-of-care virus detection device is essential for the control and surveillance of the contagious severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic. The study presented here aimed to demonstrate a solid-phase isothermal recombinase polymerase amplification coupled CRISPR-based (spRPA-CRISPR) assay for on-chip multiplexed, sensitive and visual COVID-19 DNA detection. The assay targets the SARS-CoV-2 structure protein encoded genomes and can simultaneously detect two specific genes without cross-interaction. The amplified target sequences were immobilized on the one-pot device surface and detected using the mixed Cas12a-crRNA collateral cleavage of reporter-released fluorescent signal when specific genes were recognized. The endpoint signal can be directly visualized for rapid detection of COVID-19. The system was tested with samples of a broad range of concentrations (20 to 2 × 104 copies) and showed analytical sensitivity down to 20 copies per microliter. Furthermore, a low-cost blue LED flashlight (~$12) was used to provide a visible SARS-CoV-2 detection signal of the spRPA-CRISPR assay which could be purchased online easily. Thus, our platform provides a sensitive and easy-to-read multiplexed gene detection method that can specifically identify low concentration genes.
Keywords: COVID-19; CRISPR-Based assay; DNA sensor; Multiplexed spRPA-CRISPR assay; Point-of-care; Visual detection.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


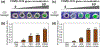

Similar articles
-
A photocontrolled one-pot isothermal amplification and CRISPR-Cas12a assay for rapid detection of SARS-CoV-2 Omicron variants.Microbiol Spectr. 2024 Mar 5;12(3):e0364523. doi: 10.1128/spectrum.03645-23. Epub 2024 Feb 6. Microbiol Spectr. 2024. PMID: 38319081 Free PMC article.
-
iSCAN-V2: A One-Pot RT-RPA-CRISPR/Cas12b Assay for Point-of-Care SARS-CoV-2 Detection.Front Bioeng Biotechnol. 2022 Jan 21;9:800104. doi: 10.3389/fbioe.2021.800104. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35127671 Free PMC article.
-
Sensitive and rapid on-site detection of SARS-CoV-2 using a gold nanoparticle-based high-throughput platform coupled with CRISPR/Cas12-assisted RT-LAMP.Sens Actuators B Chem. 2021 Oct 15;345:130411. doi: 10.1016/j.snb.2021.130411. Epub 2021 Jul 6. Sens Actuators B Chem. 2021. PMID: 34248284 Free PMC article.
-
Detection of Infectious Viruses Using CRISPR-Cas12-Based Assay.Biosensors (Basel). 2021 Aug 28;11(9):301. doi: 10.3390/bios11090301. Biosensors (Basel). 2021. PMID: 34562891 Free PMC article.
-
Reverse Transcription Recombinase Polymerase Amplification Coupled with CRISPR-Cas12a for Facile and Highly Sensitive Colorimetric SARS-CoV-2 Detection.Anal Chem. 2021 Mar 2;93(8):4126-4133. doi: 10.1021/acs.analchem.1c00013. Epub 2021 Feb 11. Anal Chem. 2021. PMID: 33570401
Cited by
-
Current Trends in RNA Virus Detection via Nucleic Acid Isothermal Amplification-Based Platforms.Biosensors (Basel). 2024 Feb 11;14(2):97. doi: 10.3390/bios14020097. Biosensors (Basel). 2024. PMID: 38392016 Free PMC article. Review.
-
Nanoplasmonic microarray-based solid-phase amplification for highly sensitive and multiplexed molecular diagnostics: application for detecting SARS-CoV-2.Mikrochim Acta. 2024 Oct 29;191(11):715. doi: 10.1007/s00604-024-06723-4. Mikrochim Acta. 2024. PMID: 39472332 Free PMC article.
References
-
- Cepheid, 2022. Package Insert, “Xpert® Xpress SARS-CoV-2. https://www.fda.gov/media/136315/download.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
