Synovial mesenchymal stem cell-derived exosomal miR-485-3p relieves cartilage damage in osteoarthritis by targeting the NRP1-mediated PI3K/Akt pathway: Exosomal miR-485-3p relieves cartilage damage
- PMID: 38293485
- PMCID: PMC10826677
- DOI: 10.1016/j.heliyon.2024.e24042
Synovial mesenchymal stem cell-derived exosomal miR-485-3p relieves cartilage damage in osteoarthritis by targeting the NRP1-mediated PI3K/Akt pathway: Exosomal miR-485-3p relieves cartilage damage
Abstract
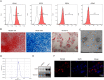
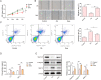
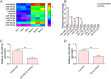
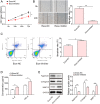
Osteoarthritis (OA) is an age-related musculoskeletal disease that results in pain and functional disability. Stem cell therapy has been considered as a promising treatment for OA. In this study, the therapeutic action and potential mechanism of synovial mesenchymal stem cells (SMSCs)-derived exosomes (Exos) in OA cartilage damage were investigated. Cartilage cells were stimulated with IL-1β to establish an in vitro model of OA cartilage damage. Cartilage cell functions were detected by CCK-8, scratch assay, and flow cytometry, respectively. Inflammatory cytokine levels were assessed by ELISA. Target molecule levels were measured by qRT‒PCR and Western blotting. Exos-induced differential expression of miRNAs in cartilage cells were analyzed by microarray analysis. The interaction between miR-485-3p and neuropilin-1 (NRP1) was validated by dual luciferase reporter and RIP assays. We found that treatment with Exos promoted proliferation, migration, and ECM secretion, but restrained apoptosis and inflammation of IL-1β-exposed cartilage cells via up-regulation of miR-485-3p. Additionally, miR-485-3p directly targeted NRP1 to repress NRP1 expression, which subsequently caused inactivation of the PI3K/Akt pathway. The protective effect of Exos on cartilage damage was counteracted by NRP1 overexpression-mediated activation of the PI3K/Akt pathway. In conclusion, Exos delivered miR-485-3p to attenuate IL-1β-induced cartilage degradation by targeting NRP1 and succedent inactivation of the PI3K/Akt pathway. Our findings shed light on the novel protective mechanism of Exos in OA, which suggest that the restoration of miR-485-3p by Exos might be a novel approach for OA treatment.
Keywords: Cartilage damage; Exos; NRP1; Osteoarthritis; miR-485-3p.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A.Stem Cell Res Ther. 2018 Sep 26;9(1):247. doi: 10.1186/s13287-018-1004-0. Stem Cell Res Ther. 2018. PMID: 30257711 Free PMC article.
-
Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA -376c-3p and targeting the WNT-beta-catenin signaling axis.Apoptosis. 2023 Apr;28(3-4):362-378. doi: 10.1007/s10495-022-01787-0. Epub 2022 Nov 17. Apoptosis. 2023. PMID: 36396777
-
Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes.Cell Biol Toxicol. 2021 Feb;37(1):85-96. doi: 10.1007/s10565-020-09559-9. Epub 2020 Oct 25. Cell Biol Toxicol. 2021. PMID: 33099657
-
Human umbilical cord mesenchymal stem cell-derived exosomal miR-199a-3p inhibits the MAPK4/NF-κB signaling pathway to relieve osteoarthritis.World J Stem Cells. 2025 Apr 26;17(4):103919. doi: 10.4252/wjsc.v17.i4.103919. World J Stem Cells. 2025. PMID: 40308884 Free PMC article.
-
Exosomes Derived From Human Urine-Derived Stem Cells Overexpressing miR-140-5p Alleviate Knee Osteoarthritis Through Downregulation of VEGFA in a Rat Model.Am J Sports Med. 2022 Mar;50(4):1088-1105. doi: 10.1177/03635465221073991. Epub 2022 Feb 18. Am J Sports Med. 2022. PMID: 35179989
Cited by
-
The status and hotspot analysis of research on extracellular vesicles and osteoarthritis: a bibliometric analysis.Front Pharmacol. 2025 Mar 31;16:1484437. doi: 10.3389/fphar.2025.1484437. eCollection 2025. Front Pharmacol. 2025. PMID: 40230694 Free PMC article. Review.
-
Regulation of ferroptosis in osteoarthritis and osteoarthritic chondrocytes by typical MicroRNAs in chondrocytes.Front Med (Lausanne). 2024 Nov 5;11:1478153. doi: 10.3389/fmed.2024.1478153. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39564502 Free PMC article. Review.
-
Research progress on exosomes from different sources in osteoarthritis and cartilage injury.J Orthop Surg Res. 2025 Jun 11;20(1):582. doi: 10.1186/s13018-025-06000-x. J Orthop Surg Res. 2025. PMID: 40495182 Free PMC article. Review.
-
Potential and challenges of utilizing exosomes in osteoarthritis therapy (Review).Int J Mol Med. 2025 Mar;55(3):43. doi: 10.3892/ijmm.2025.5484. Epub 2025 Jan 10. Int J Mol Med. 2025. PMID: 39791222 Free PMC article. Review.
-
An Update on Emerging Regenerative Medicine Applications: The Use of Extracellular Vesicles and Exosomes for the Management of Chronic Pain.Curr Pain Headache Rep. 2024 Dec;28(12):1289-1297. doi: 10.1007/s11916-024-01309-4. Epub 2024 Nov 4. Curr Pain Headache Rep. 2024. PMID: 39495409 Review.
References
-
- Yamamoto N., Szymski D., Voss A., Ishikawa H., Muraki T., Cunha R.A., Ejnisman B., Noack J., McCarty E., Mulcahey M.K., et al. Non-operative management of shoulder osteoarthritis: current concepts. J. ISAKOS. 2023;8:289–295. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

