Nanoplatform-Mediated Autophagy Regulation and Combined Anti-Tumor Therapy for Resistant Tumors
- PMID: 38293604
- PMCID: PMC10826716
- DOI: 10.2147/IJN.S445578
Nanoplatform-Mediated Autophagy Regulation and Combined Anti-Tumor Therapy for Resistant Tumors
Abstract
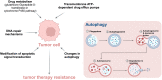
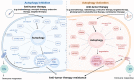
The overall cancer incidence and death toll have been increasing worldwide. However, the conventional therapies have some obvious limitations, such as non-specific targeting, systemic toxic effects, especially the multidrug resistance (MDR) of tumors, in which, autophagy plays a vital role. Therefore, there is an urgent need for new treatments to reduce adverse reactions, improve the treatment efficacy and expand their therapeutic indications more effectively and accurately. Combination therapy based on autophagy regulators is a very feasible and important method to overcome tumor resistance and sensitize anti-tumor drugs. However, the less improved efficacy, more systemic toxicity and other problems limit its clinical application. Nanotechnology provides a good way to overcome this limitation. Co-delivery of autophagy regulators combined with anti-tumor drugs through nanoplatforms provides a good therapeutic strategy for the treatment of tumors, especially drug-resistant tumors. Notably, the nanomaterials with autophagy regulatory properties have broad therapeutic prospects as carrier platforms, especially in adjuvant therapy. However, further research is still necessary to overcome the difficulties such as the safety, biocompatibility, and side effects of nanomedicine. In addition, clinical research is also indispensable to confirm its application in tumor treatment.
Keywords: autophagy; co-delivery; combination therapy; nanotechnology; tumor resistance.
© 2024 Yang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

