Combined Placental Mesenchymal Stem Cells with Guided Nanoparticles Effective Against Diabetic Nephropathy in Mouse Model
- PMID: 38293609
- PMCID: PMC10826715
- DOI: 10.2147/IJN.S446733
Combined Placental Mesenchymal Stem Cells with Guided Nanoparticles Effective Against Diabetic Nephropathy in Mouse Model
Abstract
Background: Diabetic nephropathy (DN) is a prevalent complication of diabetes mellitus and constitutes the primary cause of mortality in affected patients. Previous studies have shown that placental mesenchymal stem cells (PL-MSCs) can alleviate kidney dysfunction in animal models of DN. However, the limited ability of mesenchymal stem cells (MSCs) to home to damaged sites restricts their therapeutic potential. Enhancing the precision of PL-MSCs' homing to target tissues is therefore vital for the success of cell therapies in treating DN.
Methods: We developed Fe3O4 coated polydopamine nanoparticle (NP)-internalized MSCs and evaluated their therapeutic effectiveness in a mouse model of streptozotocin- and high-fat diet-induced DN, using an external magnetic field.
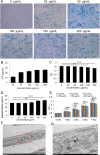
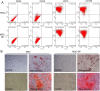
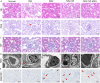
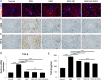
Results: Our study confirmed that NPs were effectively internalized into PL-MSCs without compromising their intrinsic stem cell properties. The magnetic targeting of PL-MSCs notably improved their homing to the kidney tissues in mice with DN, resulting in enhanced kidney function compared to the transplantation of PL-MSCs alone. Furthermore, the anti-inflammatory and antifibrotic attributes of PL-MSCs played a role in the recovery of kidney function and structure.
Conclusion: These results demonstrate that magnetically targeted therapy using PL-MSCs is a promising approach for treating diabetic nephropathy.
Keywords: diabetic nephropathy; iron oxide nanoparticles; magnetic targeting; mesenchymal stem cells.
© 2024 Wang et al.
Conflict of interest statement
Ke Wang and Te Liu contributed equally to this work and are co-first authors. The authors declare no conflicts of interest in relation to this work.
Figures




Similar articles
-
Effects of xenogeneic transplantation of umbilical cord-derived mesenchymal stem cells combined with irbesartan on renal podocyte damage in diabetic rats.Stem Cell Res Ther. 2024 Jul 30;15(1):239. doi: 10.1186/s13287-024-03844-8. Stem Cell Res Ther. 2024. PMID: 39080783 Free PMC article.
-
The Role of Mesenchymal Stem Cells in Treating Diabetic Kidney Disease: Immunomodulatory Effects and Kidney Regeneration.Int J Med Sci. 2025 Mar 3;22(7):1720-1735. doi: 10.7150/ijms.103806. eCollection 2025. Int J Med Sci. 2025. PMID: 40093796 Free PMC article.
-
Intervention for early diabetic nephropathy by mesenchymal stem cells in a preclinical nonhuman primate model.Stem Cell Res Ther. 2019 Dec 2;10(1):363. doi: 10.1186/s13287-019-1401-z. Stem Cell Res Ther. 2019. PMID: 31791397 Free PMC article.
-
The Multi-Therapeutic Role of MSCs in Diabetic Nephropathy.Front Endocrinol (Lausanne). 2021 Jun 7;12:671566. doi: 10.3389/fendo.2021.671566. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34163437 Free PMC article. Review.
-
Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy.Life Sci. 2018 Dec 15;215:113-118. doi: 10.1016/j.lfs.2018.11.001. Epub 2018 Nov 3. Life Sci. 2018. PMID: 30399376 Review.
Cited by
-
Targeting programmed cell death in diabetic kidney disease: from molecular mechanisms to pharmacotherapy.Mol Med. 2024 Dec 20;30(1):265. doi: 10.1186/s10020-024-01020-5. Mol Med. 2024. PMID: 39707216 Free PMC article. Review.
-
Mesenchymal Stem Cell Therapy: Therapeutic Opportunities and Challenges for Diabetic Kidney Disease.Int J Mol Sci. 2024 Sep 30;25(19):10540. doi: 10.3390/ijms251910540. Int J Mol Sci. 2024. PMID: 39408867 Free PMC article. Review.
-
Combined Therapy Using Mesenchymal Stem Cells and Metal Nanoparticles: Perspectives for Ocular Injuries and Diseases.Int J Nanomedicine. 2025 Jun 12;20:7403-7414. doi: 10.2147/IJN.S527928. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40529539 Free PMC article. Review.
-
[MiR-139-5p regulates the Notch/RBP-J/Hes1 axis to promote homing of bone mesenchymal stem cells in bronchial asthma].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Dec 20;44(12):2283-2290. doi: 10.12122/j.issn.1673-4254.2024.12.03. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39725616 Free PMC article. Chinese.
References
-
- Molitch ME. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–83. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

