The role of s-palmitoylation in neurological diseases: implication for zDHHC family
- PMID: 38293675
- PMCID: PMC10824933
- DOI: 10.3389/fphar.2023.1342830
The role of s-palmitoylation in neurological diseases: implication for zDHHC family
Abstract
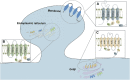
S-palmitoylation is a reversible posttranslational modification, and the palmitoylation reaction in human-derived cells is mediated by the zDHHC family, which is composed of S-acyltransferase enzymes that possess the DHHC (Asp-His-His-Cys) structural domain. zDHHC proteins form an autoacylation intermediate, which then attaches the fatty acid to cysteine a residue in the target protein. zDHHC proteins sublocalize in different neuronal structures and exert dif-ferential effects on neurons. In humans, many zDHHC proteins are closely related to human neu-rological disor-ders. This review focuses on a variety of neurological disorders, such as AD (Alz-heimer's disease), HD (Huntington's disease), SCZ (schizophrenia), XLID (X-linked intellectual disability), attention deficit hyperactivity disorder and glioma. In this paper, we will discuss and summarize the research progress regarding the role of zDHHC proteins in these neu-rological disorders.
Keywords: S-palmitoylation; glutamate receptor; neurological diseases; protein acyltransferase; scaffold protein; zDHHC.
Copyright © 2024 Liao, Huang, Liu, Zhang, Shi, Li and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Andrew R. J., Fernandez C. G., Stanley M., Jiang H., Nguyen P., Rice R. C., et al. (2017). Lack of BACE1 S-palmitoylation reduces amyloid burden and mitigates memory deficits in transgenic mouse models of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 114 (45), E9665–E9674. 10.1073/pnas.1708568114 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

