Hypoxia Attenuates Pressure Overload-Induced Heart Failure
- PMID: 38293923
- PMCID: PMC11056135
- DOI: 10.1161/JAHA.123.033553
Hypoxia Attenuates Pressure Overload-Induced Heart Failure
Abstract
Background: Alveolar hypoxia is protective in the context of cardiovascular and ischemic heart disease; however, the underlying mechanisms are incompletely understood. The present study sought to test the hypothesis that hypoxia is cardioprotective in left ventricular pressure overload (LVPO)-induced heart failure. We furthermore aimed to test that overlapping mechanisms promote cardiac recovery in heart failure patients following left ventricular assist device-mediated mechanical unloading and circulatory support.
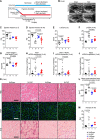
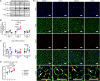
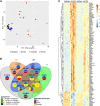
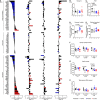
Methods and results: We established a novel murine model of combined chronic alveolar hypoxia and LVPO following transverse aortic constriction (HxTAC). The HxTAC model is resistant to cardiac hypertrophy and the development of heart failure. The cardioprotective mechanisms identified in our HxTAC model include increased activation of HIF (hypoxia-inducible factor)-1α-mediated angiogenesis, attenuated induction of genes associated with pathological remodeling, and preserved metabolic gene expression as identified by RNA sequencing. Furthermore, LVPO decreased Tbx5 and increased Hsd11b1 mRNA expression under normoxic conditions, which was attenuated under hypoxic conditions and may induce additional hypoxia-mediated cardioprotective effects. Analysis of samples from patients with advanced heart failure that demonstrated left ventricular assist device-mediated myocardial recovery revealed a similar expression pattern for TBX5 and HSD11B1 as observed in HxTAC hearts.
Conclusions: Hypoxia attenuates LVPO-induced heart failure. Cardioprotective pathways identified in the HxTAC model might also contribute to cardiac recovery following left ventricular assist device support. These data highlight the potential of our novel HxTAC model to identify hypoxia-mediated cardioprotective mechanisms and therapeutic targets that attenuate LVPO-induced heart failure and mediate cardiac recovery following mechanical circulatory support.
Keywords: cardiac hypertrophy; cardiac remodeling; hypoxia; left ventricular assist device; pressure overload.
Figures



References
-
- Faeh D, Moser A, Panczak R, Bopp M, Roosli M, Spoerri A; Swiss National Cohort Study Group . Independent at heart: persistent association of altitude with ischaemic heart disease mortality after consideration of climate, topography and built environment. J Epidemiol Community Health. 2016;70:798–806. doi: 10.1136/jech-2015-206210 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

