Cross-Linking Methods of the Silk Protein Hydrogel in Oral and Craniomaxillofacial Tissue Regeneration
- PMID: 38294593
- PMCID: PMC11087422
- DOI: 10.1007/s13770-023-00624-y
Cross-Linking Methods of the Silk Protein Hydrogel in Oral and Craniomaxillofacial Tissue Regeneration
Abstract
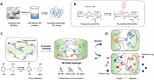
Background: Craniomaxillofacial tissue defects are clinical defects involving craniomaxillofacial and oral soft and hard tissues. They are characterized by defect-shaped irregularities, bacterial and inflammatory environments, and the need for functional recovery. Conventional clinical treatments are currently unable to achieve regeneration of high-quality oral craniomaxillofacial tissue. As a natural biomaterial, silk fibroin (SF) has been widely studied in biomedicine and has broad prospects for use in tissue regeneration. Hydrogels made of SF showed excellent water retention, biocompatibility, safety and the ability to combine with other materials.
Methods: To gain an in-depth understanding of the current development of SF, this article reviews the structure, preparation and application prospects in oral and craniomaxillofacial tissue regenerative medicine. It first briefly introduces the structure of SF and then summarizes the principles, advantages and disadvantages of the different cross-linking methods (physical cross-linking, chemical cross-linking and double network structure) of SF. Finally, the existing research on the use of SF in tissue engineering and the prospects of using SF with different cross-linking methods in oral and craniomaxillofacial tissue regeneration are also discussed.
Conclusions: This review is intended to show the advantages of SF hydrogels in tissue engineering and provides theoretical support for establishing novel and viable silk protein hydrogels for regeneration.
Keywords: Crosslinking reagents; Guided tissue regeneration; Pulp tissue; Silk fibroin.
© 2024. Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors have no conflicts of interest relevant to this study to disclose.
Figures









Similar articles
-
Progress of silk fibroin biomaterial use in oral tissue regeneration engineering.Crit Rev Biotechnol. 2025 Sep;45(6):1232-1248. doi: 10.1080/07388551.2025.2472621. Epub 2025 Mar 24. Crit Rev Biotechnol. 2025. PMID: 40125866 Review.
-
Physically Cross-Linked Silk Fibroin Hydrogel with Rapid Sol-Gel Transition and Enhanced Mechanical Performance.Macromol Rapid Commun. 2025 Jul;46(14):e2401016. doi: 10.1002/marc.202401016. Epub 2025 May 14. Macromol Rapid Commun. 2025. PMID: 40366237
-
Silk fibroin-hyaluronic acid nanofibers for peripheral nerve regeneration.J Biomater Appl. 2025 Aug;40(2):307-323. doi: 10.1177/08853282251329315. Epub 2025 Mar 25. J Biomater Appl. 2025. PMID: 40131186
-
[Research progress on silk fibroin-nerve guidance conduits for peripheral nerve injury repair].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025 Jun 15;39(6):777-782. doi: 10.7507/1002-1892.202504070. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025. PMID: 40545469 Free PMC article. Review. Chinese.
-
Silk Fibroin/GelMA-Based Hydrogels as a Platform for Tissue Adhesives and Tissue Engineering.ACS Biomater Sci Eng. 2025 Jul 14;11(7):3893-3931. doi: 10.1021/acsbiomaterials.5c00286. Epub 2025 May 30. ACS Biomater Sci Eng. 2025. PMID: 40445067 Review.
Cited by
-
Molecular Engineering of Recombinant Protein Hydrogels: Programmable Design and Biomedical Applications.Gels. 2025 Jul 26;11(8):579. doi: 10.3390/gels11080579. Gels. 2025. PMID: 40868710 Free PMC article. Review.
-
Advancements and Challenges in Hydrogel Engineering for Regenerative Medicine.Gels. 2024 Mar 30;10(4):238. doi: 10.3390/gels10040238. Gels. 2024. PMID: 38667657 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 202202130501009/Shanxi Provincial Key Research and Development Project
- 2022XM56/Shanxi Provincial Medical Key Scientific Research Project
- 202203021211225/Basic Research Program of Shanxi Province project
- 2020L0207/Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi
- KY201804/Shanxi Medical University School and Hospital of Stomatology Program
LinkOut - more resources
Full Text Sources
Research Materials
