Exploring the frontiers: tumor immune microenvironment and immunotherapy in head and neck squamous cell carcinoma
- PMID: 38294629
- PMCID: PMC10830966
- DOI: 10.1007/s12672-024-00870-z
Exploring the frontiers: tumor immune microenvironment and immunotherapy in head and neck squamous cell carcinoma
Abstract
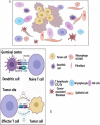
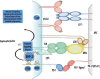
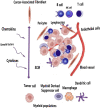
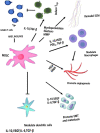
The global prevalence of head and neck malignancies positions them as the sixth most common form of cancer, with the head and neck squamous cell carcinoma (HNSCC) representing the predominant histological subtype. Despite advancements in multidisciplinary approaches and molecular targeted therapies, the therapeutic outcomes for HNSCC have only marginally improved, particularly in cases of recurrent or metastatic HNSCC (R/MHNSCC). This situation underscores the critical necessity for the development of innovative therapeutic strategies. Such strategies are essential not only to enhance the efficacy of HNSCC treatment but also to minimize the incidence of associated complications, thus improving overall patient prognosis. Cancer immunotherapy represents a cutting-edge cancer treatment that leverages the immune system for targeting and destroying cancer cells. It's applied to multiple cancers, including melanoma and lung cancer, offering precision, adaptability, and the potential for long-lasting remission through immune memory. It is observed that while HNSCC patients responsive to immunotherapy often experience prolonged therapeutic benefits, only a limited subset demonstrates such responsiveness. Additionally, significant clinical challenges remain, including the development of resistance to immunotherapy. The biological characteristics, dynamic inhibitory changes, and heterogeneity of the tumor microenvironment (TME) in HNSCC play critical roles in its pathogenesis, immune evasion, and therapeutic resistance. This review aims to elucidate the functions and mechanisms of anti-tumor immune cells and extracellular components within the HNSCC TME. It also introduces several immunosuppressive agents commonly utilized in HNSCC immunotherapy, examines factors influencing the effectiveness of these treatments, and provides a comprehensive summary of immunotherapeutic strategies relevant to HNSCC.
Keywords: Head and neck squamous cell carcinoma; Immunotherapy; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
Grants and funding
- No. 2020YFB1312805/National Key R&D Program of China
- No.2022-1-2051/The Capital Health Research and Development of Special
- No. 82002880/National Natural Science Foundation of China
- PX2021008/Beijing Municipal Administration of Hospitals Incubating Program
- QML20200205/Beijing Hospitals Authority Youth Programme
LinkOut - more resources
Full Text Sources
