A Novel Method to Repair Thin Endometrium and Restore Fertility Based on Menstruation-Derived Stem Cell
- PMID: 38294669
- PMCID: PMC11111544
- DOI: 10.1007/s43032-024-01458-2
A Novel Method to Repair Thin Endometrium and Restore Fertility Based on Menstruation-Derived Stem Cell
Abstract
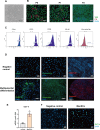
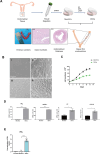
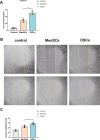
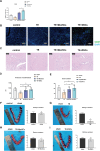
Thin endometrium (TE), which mainly occurs as a result of severe damage to the endometrial basalis, is one of the prominent etiologies of menstrual abnormalities, infertility, and recurrent miscarriage in women. Previous studies have demonstrated that mesenchymal stem cells (MSCs) are considered ideal cells with multipotency for regenerative medicine and exhibit therapeutic effects on TE through their cellular secretions. However, there is limited research on strategies to enhance MSC secretion to improve their therapeutic efficacy. Herein, we isolated menstrual blood-derived mesenchymal stem cells (MenSCs) from menstruation and transformed them into decidualized stromal cells (DSCs), which are specialized cells with enhanced secretory functions. To assess the therapeutic potential of DSCs compared to MenSCs, we conducted a series of experiments in cells and animals. The results demonstrated that DSCs exhibited changes in morphology compared to MenSCs, with a decrease in cell proliferation but a significant improvement in secretion function. Furthermore, DSCs facilitated the restoration of endometrial thickness and increased the number of glands and blood vessel formation. Most importantly, the pregnancy rates in rats were effectively restored, bringing them closer to normal levels. These findings greatly contribute to our understanding of stem cell therapy for TE and strongly suggest that DSCs could hold significant promise as a potential treatment option for TE.
Keywords: Animal model; Decidualization; Menstrual blood stem cells (MenSCs); Stem cell therapy; Thin endometrium.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways.Reproduction. 2016 Nov;152(5):389-402. doi: 10.1530/REP-16-0286. Epub 2016 Aug 2. Reproduction. 2016. PMID: 27486270
-
Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion.Stem Cell Res Ther. 2019 Feb 15;10(1):61. doi: 10.1186/s13287-019-1155-7. Stem Cell Res Ther. 2019. PMID: 30770774 Free PMC article.
-
MALAT1/miR-7-5p/TCF4 Axis Regulating Menstrual Blood Mesenchymal Stem Cells Improve Thin Endometrium Fertility by the Wnt Signaling Pathway.Cell Transplant. 2024 Jan-Dec;33:9636897241259552. doi: 10.1177/09636897241259552. Cell Transplant. 2024. PMID: 38847385 Free PMC article.
-
Steps towards the clinical application of endometrial and menstrual fluid mesenchymal stem cells for the treatment of gynecological disorders.Expert Opin Biol Ther. 2025 Mar;25(3):285-307. doi: 10.1080/14712598.2025.2465826. Epub 2025 Feb 19. Expert Opin Biol Ther. 2025. PMID: 39925343 Review.
-
Adult stem cells in endometrial regeneration: Molecular insights and clinical applications.Mol Reprod Dev. 2021 Jun;88(6):379-394. doi: 10.1002/mrd.23476. Epub 2021 May 20. Mol Reprod Dev. 2021. PMID: 34014590 Free PMC article. Review.
Cited by
-
New advances in the treatment of thin endometrium.Front Endocrinol (Lausanne). 2024 Apr 30;15:1269382. doi: 10.3389/fendo.2024.1269382. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38745960 Free PMC article.
-
Long-term therapeutic effects of allogeneic mesenchymal stem cell transplantation for intrauterine adhesions.Stem Cell Res Ther. 2024 Dec 23;15(1):499. doi: 10.1186/s13287-024-04100-9. Stem Cell Res Ther. 2024. PMID: 39716301 Free PMC article.
-
Body Fluid-Derived Stem Cells: Powering Innovative, Less-Invasive Cell Therapies.Int J Mol Sci. 2025 May 5;26(9):4382. doi: 10.3390/ijms26094382. Int J Mol Sci. 2025. PMID: 40362618 Free PMC article. Review.
-
Stem cell treatments for female reproductive disorders: a comprehensive review.J Ovarian Res. 2025 Jul 24;18(1):161. doi: 10.1186/s13048-025-01750-y. J Ovarian Res. 2025. PMID: 40708035 Free PMC article. Review.
-
Therapeutic potential of super activated platelet lysate (sPL) and umbilical cord mesenchymal stem cells (UCMSCs) in enhancing endometrial regeneration in rats with thin endometrium.Sci Rep. 2025 Jul 9;15(1):24698. doi: 10.1038/s41598-025-10587-w. Sci Rep. 2025. PMID: 40634493 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

