Targeting prostate tumor low-molecular weight tyrosine phosphatase for oxidation-sensitizing therapy
- PMID: 38295166
- PMCID: PMC10830117
- DOI: 10.1126/sciadv.adg7887
Targeting prostate tumor low-molecular weight tyrosine phosphatase for oxidation-sensitizing therapy
Abstract
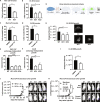
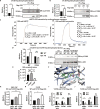
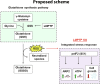
Protein tyrosine phosphatases (PTPs) play major roles in cancer and are emerging as therapeutic targets. Recent reports suggest low-molecular weight PTP (LMPTP)-encoded by the ACP1 gene-is overexpressed in prostate tumors. We found ACP1 up-regulated in human prostate tumors and ACP1 expression inversely correlated with overall survival. Using CRISPR-Cas9-generated LMPTP knockout C4-2B and MyC-CaP cells, we identified LMPTP as a critical promoter of prostate cancer (PCa) growth and bone metastasis. Through metabolomics, we found that LMPTP promotes PCa cell glutathione synthesis by dephosphorylating glutathione synthetase on inhibitory Tyr270. PCa cells lacking LMPTP showed reduced glutathione, enhanced activation of eukaryotic initiation factor 2-mediated stress response, and enhanced reactive oxygen species after exposure to taxane drugs. LMPTP inhibition slowed primary and bone metastatic prostate tumor growth in mice. These findings reveal a role for LMPTP as a critical promoter of PCa growth and metastasis and validate LMPTP inhibition as a therapeutic strategy for treating PCa through sensitization to oxidative stress.
Figures



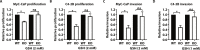


References
-
- Siegel R. L., Miller K. D., Jemal A., Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019). - PubMed
-
- Pignot G., Maillet D., Gross E., Barthelemy P., Beauval J.-B., Constans-Schlurmann F., Loriot Y., Ploussard G., Sargos P., Timsit M.-O., Vincendeau S., Pasticier G., Borchiellini D., Systemic treatments for high-risk localized prostate cancer. Nat. Rev. Urol. 15, 498–510 (2018). - PubMed
-
- Oster G., Lamerato L., Glass A. G., Richert-Boe K. E., Lopez A., Chung K., Richhariya A., Dodge T., Wolff G. G., Balakumaran A., Edelsberg J., Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: A 15-year study in two large US health systems. Support. Care Cancer 21, 3279–3286 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

