Regulatory T cells suppress myeloma-specific immunity during autologous stem cell mobilization and transplantation
- PMID: 38295333
- PMCID: PMC11103090
- DOI: 10.1182/blood.2023022000
Regulatory T cells suppress myeloma-specific immunity during autologous stem cell mobilization and transplantation
Abstract
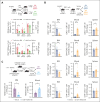
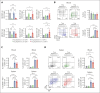
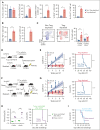
Autologous stem cell transplantation (ASCT) is the standard of care consolidation therapy for eligible patients with myeloma but most patients eventually progress, an event associated with features of immune escape. Novel approaches to enhance antimyeloma immunity after ASCT represent a major unmet need. Here, we demonstrate that patient-mobilized stem cell grafts contain high numbers of effector CD8 T cells and immunosuppressive regulatory T cells (Tregs). We showed that bone marrow (BM)-residing T cells are efficiently mobilized during stem cell mobilization (SCM) and hypothesized that mobilized and highly suppressive BM-derived Tregs might limit antimyeloma immunity during SCM. Thus, we performed ASCT in a preclinical myeloma model with or without stringent Treg depletion during SCM. Treg depletion generated SCM grafts containing polyfunctional CD8 T effector memory cells, which dramatically enhanced myeloma control after ASCT. Thus, we explored clinically tractable translational approaches to mimic this scenario. Antibody-based approaches resulted in only partial Treg depletion and were inadequate to recapitulate this effect. In contrast, a synthetic interleukin-2 (IL-2)/IL-15 mimetic that stimulates the IL-2 receptor on CD8 T cells without binding to the high-affinity IL-2Ra used by Tregs efficiently expanded polyfunctional CD8 T cells in mobilized grafts and protected recipients from myeloma progression after ASCT. We confirmed that Treg depletion during stem cell mobilization can mitigate constraints on tumor immunity and result in profound myeloma control after ASCT. Direct and selective cytokine signaling of CD8 T cells can recapitulate this effect and represent a clinically testable strategy to improve responses after ASCT.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: D.J.G. has received research funding from Bristol Myers Squibb, Cellectar Biosciences, Janssen Biotech, Juno Therapeutics, Seattle Genetics, and Springworks Therapeutics; consulted for Legend Biotech; is a member of the board or advisory committee for Bristol Myers Squibb, GSK, Janssen Biotech, Neoleukin Therapeutics, and Seattle Genetics; and has patents for Juno therapeutics. G.R.H. has consulted for Generon Corporation, NapaJen Pharma, iTeos Therapeutics, Neoleukin Therapeutics, Commonwealth Serum Laboratories, and Cynata Therapeutics and has received research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, Serplus Technology, Heat Biologics, Laevoroc Oncology, iTEOS therapeutics, and Genentech. The remaining authors declare no competing financial interests.
Figures




Comment in
-
Treg depletion supercharges ASCT power.Blood. 2024 Apr 18;143(16):1562-1563. doi: 10.1182/blood.2024024075. Blood. 2024. PMID: 38635248 No abstract available.
References
-
- Huang J, Chan SC, Lok V, et al. The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022;9(9):e670–e677. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

