Reactive oxygen species generation by reverse electron transfer at mitochondrial complex I under simulated early reperfusion conditions
- PMID: 38295577
- PMCID: PMC10844975
- DOI: 10.1016/j.redox.2024.103047
Reactive oxygen species generation by reverse electron transfer at mitochondrial complex I under simulated early reperfusion conditions
Abstract
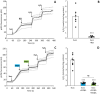
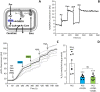
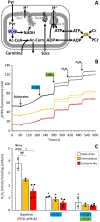
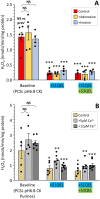
Ischemic tissues accumulate succinate, which is rapidly oxidized upon reperfusion, driving a burst of mitochondrial reactive oxygen species (ROS) generation that triggers cell death. In isolated mitochondria with succinate as the sole metabolic substrate under non-phosphorylating conditions, 90 % of ROS generation is from reverse electron transfer (RET) at the Q site of respiratory complex I (Cx-I). Together, these observations suggest Cx-I RET is the source of pathologic ROS in reperfusion injury. However, numerous factors present in early reperfusion may impact Cx-I RET, including: (i) High [NADH]; (ii) High [lactate]; (iii) Mildly acidic pH; (iv) Defined ATP/ADP ratios; (v) Presence of the nucleosides adenosine and inosine; and (vi) Defined free [Ca2+]. Herein, experiments with mouse cardiac mitochondria revealed that under simulated early reperfusion conditions including these factors, total mitochondrial ROS generation was only 56 ± 17 % of that seen with succinate alone (mean ± 95 % confidence intervals). Of this ROS, only 52 ± 20 % was assignable to Cx-I RET. A further 14 ± 7 % could be assigned to complex III, with the remainder (34 ± 11 %) likely originating from other ROS sources upstream of the Cx-I Q site. Together, these data suggest the relative contribution of Cx-I RET ROS to reperfusion injury may be overestimated, and other ROS sources may contribute a significant fraction of ROS in early reperfusion.
Keywords: Complex-I; Ischemia; Mitochondria; Reactive oxygen species; Reperfusion; Reverse electron transfer.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors hereby state that they have no conflicts of interest, financial or otherwise, regarding the content of this paper.
Figures

Update of
-
Reactive Oxygen Species Generation by Reverse Electron Transfer at Mitochondrial Complex I Under Simulated Early Reperfusion Conditions.bioRxiv [Preprint]. 2023 Nov 21:2023.11.21.568136. doi: 10.1101/2023.11.21.568136. bioRxiv. 2023. Update in: Redox Biol. 2024 Apr;70:103047. doi: 10.1016/j.redox.2024.103047. PMID: 38045326 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

