Large-scale duplication events underpin population-level flexibility in tRNA gene copy number in Pseudomonas fluorescens SBW25
- PMID: 38296823
- PMCID: PMC10954465
- DOI: 10.1093/nar/gkae049
Large-scale duplication events underpin population-level flexibility in tRNA gene copy number in Pseudomonas fluorescens SBW25
Abstract
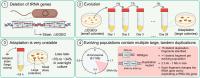
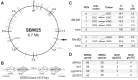
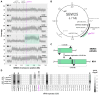
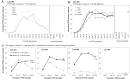
The complement of tRNA genes within a genome is typically considered to be a (relatively) stable characteristic of an organism. Here, we demonstrate that bacterial tRNA gene set composition can be more flexible than previously appreciated, particularly regarding tRNA gene copy number. We report the high-rate occurrence of spontaneous, large-scale, tandem duplication events in laboratory populations of the bacterium Pseudomonas fluorescens SBW25. The identified duplications are up to ∼1 Mb in size (∼15% of the wildtype genome) and are predicted to change the copy number of up to 917 genes, including several tRNA genes. The observed duplications are inherently unstable: they occur, and are subsequently lost, at extremely high rates. We propose that this unusually plastic type of mutation provides a mechanism by which tRNA gene set diversity can be rapidly generated, while simultaneously preserving the underlying tRNA gene set in the absence of continued selection. That is, if a tRNA set variant provides no fitness advantage, then high-rate segregation of the duplication ensures the maintenance of the original tRNA gene set. However, if a tRNA gene set variant is beneficial, the underlying duplication fragment(s) may persist for longer and provide raw material for further, more stable, evolutionary change.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
The birth of a bacterial tRNA gene by large-scale, tandem duplication events.Elife. 2020 Oct 30;9:e57947. doi: 10.7554/eLife.57947. Elife. 2020. PMID: 33124983 Free PMC article.
-
Genomic, genetic and structural analysis of pyoverdine-mediated iron acquisition in the plant growth-promoting bacterium Pseudomonas fluorescens SBW25.BMC Microbiol. 2008 Jan 14;8:7. doi: 10.1186/1471-2180-8-7. BMC Microbiol. 2008. PMID: 18194565 Free PMC article.
-
Genetic characterization of Pseudomonas fluorescens SBW25 rsp gene expression in the phytosphere and in vitro.J Bacteriol. 2005 Dec;187(24):8477-88. doi: 10.1128/JB.187.24.8477-8488.2005. J Bacteriol. 2005. PMID: 16321952 Free PMC article.
-
Copy-number changes in evolution: rates, fitness effects and adaptive significance.Front Genet. 2013 Dec 10;4:273. doi: 10.3389/fgene.2013.00273. Front Genet. 2013. PMID: 24368910 Free PMC article. Review.
-
Role of selection in fixation of gene duplications.J Theor Biol. 2006 Mar 21;239(2):141-51. doi: 10.1016/j.jtbi.2005.08.033. Epub 2005 Oct 20. J Theor Biol. 2006. PMID: 16242725 Review.
Cited by
-
Genome engineering on size reduction and complexity simplification: A review.J Adv Res. 2024 Jun;60:159-171. doi: 10.1016/j.jare.2023.07.006. Epub 2023 Jul 12. J Adv Res. 2024. PMID: 37442424 Free PMC article. Review.
-
Antibiotic resistance mediated by gene amplifications.NPJ Antimicrob Resist. 2024 Nov 6;2(1):35. doi: 10.1038/s44259-024-00052-5. NPJ Antimicrob Resist. 2024. PMID: 39843582 Free PMC article. Review.
-
A more significant role for insertion sequences in large-scale rearrangements in bacterial genomes.mBio. 2025 Jan 8;16(1):e0305224. doi: 10.1128/mbio.03052-24. Epub 2024 Dec 5. mBio. 2025. PMID: 39636122 Free PMC article.
References
-
- Rak R., Dahan O., Pilpel Y. Repertoires of tRNAs: the couplers of genomics and proteomics. Annu. Rev. Cell Dev. Biol. 2018; 34:239–264. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

