Hydrogel dressings with intrinsic antibiofilm and antioxidative dual functionalities accelerate infected diabetic wound healing
- PMID: 38296937
- PMCID: PMC10830466
- DOI: 10.1038/s41467-024-44968-y
Hydrogel dressings with intrinsic antibiofilm and antioxidative dual functionalities accelerate infected diabetic wound healing
Erratum in
-
Author Correction: Hydrogel dressings with intrinsic antibiofilm and antioxidative dual functionalities accelerate infected diabetic wound healing.Nat Commun. 2025 Oct 10;16(1):9047. doi: 10.1038/s41467-025-64329-7. Nat Commun. 2025. PMID: 41073442 Free PMC article. No abstract available.
Abstract
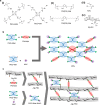
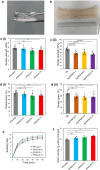
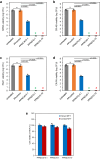
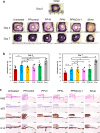
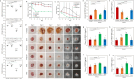
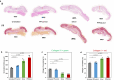
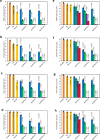
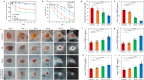
Chronic wounds are often infected with biofilm bacteria and characterized by high oxidative stress. Current dressings that promote chronic wound healing either require additional processes such as photothermal irradiation or leave behind gross amounts of undesirable residues. We report a dual-functionality hydrogel dressing with intrinsic antibiofilm and antioxidative properties that are synergistic and low-leaching. The hydrogel is a crosslinked network with tethered antibacterial cationic polyimidazolium and antioxidative N-acetylcysteine. In a murine diabetic wound model, the hydrogel accelerates the closure of wounds infected with methicillin-resistant Staphylococcus aureus or carbapenem-resistant Pseudomonas aeruginosa biofilm. Furthermore, a three-dimensional ex vivo human skin equivalent model shows that N-acetylcysteine promotes the keratinocyte differentiation and accelerates the re-epithelialization process. Our hydrogel dressing can be made into different formats for the healing of both flat and deep infected chronic wounds without contamination of the wound or needing other modalities such as photothermal irradiation.
© 2024. The Author(s).
Conflict of interest statement
D.P., C.K.Y., Y.W., X.X., N.S.T., and M.B.C.-P. have filed the Singapore provisional patents for this work under the patent numbers 10202250243U and 10202301624S. C.K.Y., S.H.M., N.S.T., and M.B.C.-P. have filed a patent for this work under the patent number US20210244846A1. D.P., C.K.Y, Y.W., X.X., S.H.M, N.S.T, and M.B.C.-P. declare no other competing interests. C.F., Y.S.Y., M.I.G.V., P.L.K.L., L.Y., P.T.H., and D.I.L. declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
- H17/01/a0/009/Agency for Science, Technology and Research (A*STAR)
- H17/01/a0/0B9/Agency for Science, Technology and Research (A*STAR)
- H17/01/a0/0M9/Agency for Science, Technology and Research (A*STAR)
- H19/01/a0/OY9/Agency for Science, Technology and Research (A*STAR)
- H17/01/a0/004/Agency for Science, Technology and Research (A*STAR)
LinkOut - more resources
Full Text Sources
Medical

