Single nuclei transcriptomics in human and non-human primate striatum in opioid use disorder
- PMID: 38296993
- PMCID: PMC10831093
- DOI: 10.1038/s41467-024-45165-7
Single nuclei transcriptomics in human and non-human primate striatum in opioid use disorder
Abstract
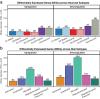
In brain, the striatum is a heterogenous region involved in reward and goal-directed behaviors. Striatal dysfunction is linked to psychiatric disorders, including opioid use disorder (OUD). Striatal subregions are divided based on neuroanatomy, each with unique roles in OUD. In OUD, the dorsal striatum is involved in altered reward processing, formation of habits, and development of negative affect during withdrawal. Using single nuclei RNA-sequencing, we identified both canonical (e.g., dopamine receptor subtype) and less abundant cell populations (e.g., interneurons) in human dorsal striatum. Pathways related to neurodegeneration, interferon response, and DNA damage were significantly enriched in striatal neurons of individuals with OUD. DNA damage markers were also elevated in striatal neurons of opioid-exposed rhesus macaques. Sex-specific molecular differences in glial cell subtypes associated with chronic stress were found in OUD, particularly female individuals. Together, we describe different cell types in human dorsal striatum and identify cell type-specific alterations in OUD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Spencer M. R., Minino A. M. & Warner M. Drug Overdose Deaths in the United States, 2001-2021. NCHSData Brief, 1–8 (2022). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

