Single-neuronal elements of speech production in humans
- PMID: 38297120
- PMCID: PMC10866697
- DOI: 10.1038/s41586-023-06982-w
Single-neuronal elements of speech production in humans
Abstract
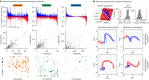
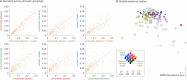
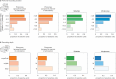
Humans are capable of generating extraordinarily diverse articulatory movement combinations to produce meaningful speech. This ability to orchestrate specific phonetic sequences, and their syllabification and inflection over subsecond timescales allows us to produce thousands of word sounds and is a core component of language1,2. The fundamental cellular units and constructs by which we plan and produce words during speech, however, remain largely unknown. Here, using acute ultrahigh-density Neuropixels recordings capable of sampling across the cortical column in humans, we discover neurons in the language-dominant prefrontal cortex that encoded detailed information about the phonetic arrangement and composition of planned words during the production of natural speech. These neurons represented the specific order and structure of articulatory events before utterance and reflected the segmentation of phonetic sequences into distinct syllables. They also accurately predicted the phonetic, syllabic and morphological components of upcoming words and showed a temporally ordered dynamic. Collectively, we show how these mixtures of cells are broadly organized along the cortical column and how their activity patterns transition from articulation planning to production. We also demonstrate how these cells reliably track the detailed composition of consonant and vowel sounds during perception and how they distinguish processes specifically related to speaking from those related to listening. Together, these findings reveal a remarkably structured organization and encoding cascade of phonetic representations by prefrontal neurons in humans and demonstrate a cellular process that can support the production of speech.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
-
- Levelt, W. J. M., Roelofs, A. & Meyer, A. S. A Theory of Lexical Access in Speech Production Vol. 22 (Cambridge Univ. Press, 1999). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

