Matrix viscoelasticity promotes liver cancer progression in the pre-cirrhotic liver
- PMID: 38297127
- PMCID: PMC10866704
- DOI: 10.1038/s41586-023-06991-9
Matrix viscoelasticity promotes liver cancer progression in the pre-cirrhotic liver
Abstract
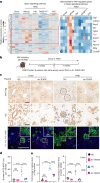
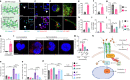
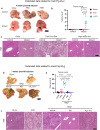
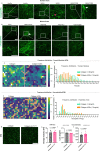
Type 2 diabetes mellitus is a major risk factor for hepatocellular carcinoma (HCC). Changes in extracellular matrix (ECM) mechanics contribute to cancer development1,2, and increased stiffness is known to promote HCC progression in cirrhotic conditions3,4. Type 2 diabetes mellitus is characterized by an accumulation of advanced glycation end-products (AGEs) in the ECM; however, how this affects HCC in non-cirrhotic conditions is unclear. Here we find that, in patients and animal models, AGEs promote changes in collagen architecture and enhance ECM viscoelasticity, with greater viscous dissipation and faster stress relaxation, but not changes in stiffness. High AGEs and viscoelasticity combined with oncogenic β-catenin signalling promote HCC induction, whereas inhibiting AGE production, reconstituting the AGE clearance receptor AGER1 or breaking AGE-mediated collagen cross-links reduces viscoelasticity and HCC growth. Matrix analysis and computational modelling demonstrate that lower interconnectivity of AGE-bundled collagen matrix, marked by shorter fibre length and greater heterogeneity, enhances viscoelasticity. Mechanistically, animal studies and 3D cell cultures show that enhanced viscoelasticity promotes HCC cell proliferation and invasion through an integrin-β1-tensin-1-YAP mechanotransductive pathway. These results reveal that AGE-mediated structural changes enhance ECM viscoelasticity, and that viscoelasticity can promote cancer progression in vivo, independent of stiffness.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
W.F., N.J.T. and O.C. are listed as inventors on a patent (WO/2023/102546) related to this this research. The other authors declare no competing interests.
Figures






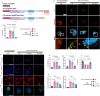
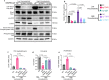
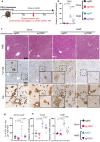
References
MeSH terms
Substances
Grants and funding
- R01 GM126256/GM/NIGMS NIH HHS/United States
- R35 GM136226/GM/NIGMS NIH HHS/United States
- I01 BX002418/BX/BLRD VA/United States
- R01 CA251155/CA/NCI NIH HHS/United States
- R01 CA277710/CA/NCI NIH HHS/United States
- R01 DK062277/DK/NIDDK NIH HHS/United States
- P30 DK120531/DK/NIDDK NIH HHS/United States
- R01 DK083283/DK/NIDDK NIH HHS/United States
- R01 CA204586/CA/NCI NIH HHS/United States
- P30 DK116074/DK/NIDDK NIH HHS/United States
- R37 CA214136/CA/NCI NIH HHS/United States
- P30 CA124435/CA/NCI NIH HHS/United States
- R01 AG060726/AG/NIA NIH HHS/United States
- S10 OD010580/OD/NIH HHS/United States
- R01 CA250227/CA/NCI NIH HHS/United States
- S10 OD032300/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

