Integrative analysis of spatial and single-cell transcriptome data from human pancreatic cancer reveals an intermediate cancer cell population associated with poor prognosis
- PMID: 38297291
- PMCID: PMC10832111
- DOI: 10.1186/s13073-024-01287-7
Integrative analysis of spatial and single-cell transcriptome data from human pancreatic cancer reveals an intermediate cancer cell population associated with poor prognosis
Abstract
Background: Recent studies using single-cell transcriptomic analysis have reported several distinct clusters of neoplastic epithelial cells and cancer-associated fibroblasts in the pancreatic cancer tumor microenvironment. However, their molecular characteristics and biological significance have not been clearly elucidated due to intra- and inter-tumoral heterogeneity.
Methods: We performed single-cell RNA sequencing using enriched non-immune cell populations from 17 pancreatic tumor tissues (16 pancreatic cancer and one high-grade dysplasia) and generated paired spatial transcriptomic data from seven patient samples.
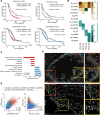
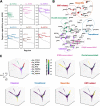
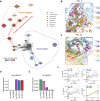
Results: We identified five distinct functional subclusters of pancreatic cancer cells and six distinct cancer-associated fibroblast subclusters. We deeply profiled their characteristics, and we found that these subclusters successfully deconvoluted most of the features suggested in bulk transcriptome analysis of pancreatic cancer. Among those subclusters, we identified a novel cancer cell subcluster, Ep_VGLL1, showing intermediate characteristics between the extremities of basal-like and classical dichotomy, despite its prognostic value. Molecular features of Ep_VGLL1 suggest its transitional properties between basal-like and classical subtypes, which is supported by spatial transcriptomic data.
Conclusions: This integrative analysis not only provides a comprehensive landscape of pancreatic cancer and fibroblast population, but also suggests a novel insight to the dynamic states of pancreatic cancer cells and unveils potential therapeutic targets.
Keywords: Cancer-associated fibroblasts; Molecular subtype of pancreatic cancer; Pancreatic cancer; Pancreatic cancer cells; Transitional cell state.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2021). Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. [cited 2021 Feb 25].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

