Human myofiber-enriched aging-induced lncRNA FRAIL1 promotes loss of skeletal muscle function
- PMID: 38297807
- PMCID: PMC11019130
- DOI: 10.1111/acel.14097
Human myofiber-enriched aging-induced lncRNA FRAIL1 promotes loss of skeletal muscle function
Abstract
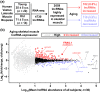
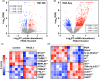
The loss of skeletal muscle mass during aging is a significant health concern linked to adverse outcomes in older individuals. Understanding the molecular basis of age-related muscle loss is crucial for developing strategies to combat this debilitating condition. Long noncoding RNAs (lncRNAs) are a largely uncharacterized class of biomolecules that have been implicated in cellular homeostasis and dysfunction across a many tissues and cell types. To identify lncRNAs that might contribute to skeletal muscle aging, we screened for lncRNAs whose expression was altered in vastus lateralis muscle from older compared to young adults. We identified FRAIL1 as an aging-induced lncRNA with high abundance in human skeletal muscle. In healthy young and older adults, skeletal muscle FRAIL1 was increased with age in conjunction with lower muscle function. Forced expression of FRAIL1 in mouse tibialis anterior muscle elicits a dose-dependent reduction in skeletal muscle fiber size that is independent of changes in muscle fiber type. Furthermore, this reduction in muscle size is dependent on an intact region of FRAIL1 that is highly conserved across non-human primates. Unbiased transcriptional and proteomic profiling of the effects of FRAIL1 expression in mouse skeletal muscle revealed widespread changes in mRNA and protein abundance that recapitulate age-related changes in pathways and processes that are known to be altered in aging skeletal muscle. Taken together, these findings shed light on the intricate molecular mechanisms underlying skeletal muscle aging and implicate FRAIL1 in age-related skeletal muscle phenotypes.
Keywords: aging; lncRNA; skeletal muscle.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could influence this work.
Figures





Similar articles
-
Exploring the lncRNAs Related to Skeletal Muscle Fiber Types and Meat Quality Traits in Pigs.Genes (Basel). 2020 Aug 4;11(8):883. doi: 10.3390/genes11080883. Genes (Basel). 2020. PMID: 32759632 Free PMC article.
-
Integrated analysis of long non-coding RNAs (lncRNAs) and mRNA expression profiles identifies lncRNA PRKG1-AS1 playing important roles in skeletal muscle aging.Aging (Albany NY). 2021 May 29;13(11):15044-15060. doi: 10.18632/aging.203067. Epub 2021 May 29. Aging (Albany NY). 2021. PMID: 34051073 Free PMC article.
-
Lnc-mg is a long non-coding RNA that promotes myogenesis.Nat Commun. 2017 Mar 10;8:14718. doi: 10.1038/ncomms14718. Nat Commun. 2017. PMID: 28281528 Free PMC article.
-
[Regulatory mechanism for lncRNAs in skeletal muscle development and progress on its research in domestic animals].Yi Chuan. 2018 Apr 20;40(4):292-304. doi: 10.16288/j.yczz.17-358. Yi Chuan. 2018. PMID: 29704375 Review. Chinese.
-
Noncoding RNAs, Emerging Regulators of Skeletal Muscle Development and Diseases.Biomed Res Int. 2015;2015:676575. doi: 10.1155/2015/676575. Epub 2015 Jul 14. Biomed Res Int. 2015. PMID: 26258142 Free PMC article. Review.
Cited by
-
LncRNA GTL2 regulates myoblast proliferation and differentiation via the PKA-CREB pathway in Duolang sheep.Zool Res. 2024 Nov 18;45(6):1261-1275. doi: 10.24272/j.issn.2095-8137.2024.125. Zool Res. 2024. PMID: 39397245 Free PMC article.
-
Network-based modelling reveals cell-type enriched patterns of non-coding RNA regulation during human skeletal muscle remodelling.NAR Mol Med. 2024 Oct 22;1(4):ugae016. doi: 10.1093/narmme/ugae016. eCollection 2024 Oct. NAR Mol Med. 2024. PMID: 39669123 Free PMC article.
-
Network-based modelling reveals cell-type enriched patterns of non-coding RNA regulation during human skeletal muscle remodelling.bioRxiv [Preprint]. 2024 Oct 9:2024.08.11.606848. doi: 10.1101/2024.08.11.606848. bioRxiv. 2024. Update in: NAR Mol Med. 2024 Oct 22;1(4):ugae016. doi: 10.1093/narmme/ugae016. PMID: 39416175 Free PMC article. Updated. Preprint.
References
-
- Afgan, E. , Baker, D. , Batut, B. , van den Beek, M. , Bouvier, D. , Cech, M. , Chilton, J. , Clements, D. , Coraor, N. , Grüning, B. A. , Guerler, A. , Hillman‐Jackson, J. , Hiltemann, S. , Jalili, V. , Rasche, H. , Soranzo, N. , Goecks, J. , Taylor, J. , Nekrutenko, A. , & Blankenberg, D. (2018). The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research, 46, W537–w544. 10.1093/nar/gky379 - DOI - PMC - PubMed
-
- Armani, A. , Galli, S. , Giacomello, E. , Bagnato, P. , Barone, V. , Rossi, D. , & Sorrentino, V. (2006). Molecular interactions with obscurin are involved in the localization of muscle‐specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Experimental Cell Research, 312, 3546–3558. 10.1016/j.yexcr.2006.07.027 - DOI - PubMed
-
- Avelar, R. A. , Ortega, J. G. , Tacutu, R. , Tyler, E. J. , Bennett, D. , Binetti, P. , Budovsky, A. , Chatsirisupachai, K. , Johnson, E. , Murray, A. , Shields, S. , Tejada‐Martinez, D. , Thornton, D. , Fraifeld, V. E. , Bishop, C. L. , & de Magalhães, J. P. (2020). A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biology, 21, 91. 10.1186/s13059-020-01990-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

