Human myofiber-enriched aging-induced lncRNA FRAIL1 promotes loss of skeletal muscle function
- PMID: 38297807
- PMCID: PMC11019130
- DOI: 10.1111/acel.14097
Human myofiber-enriched aging-induced lncRNA FRAIL1 promotes loss of skeletal muscle function
Abstract
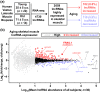
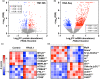
The loss of skeletal muscle mass during aging is a significant health concern linked to adverse outcomes in older individuals. Understanding the molecular basis of age-related muscle loss is crucial for developing strategies to combat this debilitating condition. Long noncoding RNAs (lncRNAs) are a largely uncharacterized class of biomolecules that have been implicated in cellular homeostasis and dysfunction across a many tissues and cell types. To identify lncRNAs that might contribute to skeletal muscle aging, we screened for lncRNAs whose expression was altered in vastus lateralis muscle from older compared to young adults. We identified FRAIL1 as an aging-induced lncRNA with high abundance in human skeletal muscle. In healthy young and older adults, skeletal muscle FRAIL1 was increased with age in conjunction with lower muscle function. Forced expression of FRAIL1 in mouse tibialis anterior muscle elicits a dose-dependent reduction in skeletal muscle fiber size that is independent of changes in muscle fiber type. Furthermore, this reduction in muscle size is dependent on an intact region of FRAIL1 that is highly conserved across non-human primates. Unbiased transcriptional and proteomic profiling of the effects of FRAIL1 expression in mouse skeletal muscle revealed widespread changes in mRNA and protein abundance that recapitulate age-related changes in pathways and processes that are known to be altered in aging skeletal muscle. Taken together, these findings shed light on the intricate molecular mechanisms underlying skeletal muscle aging and implicate FRAIL1 in age-related skeletal muscle phenotypes.
Keywords: aging; lncRNA; skeletal muscle.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could influence this work.
Figures





References
-
- Afgan, E. , Baker, D. , Batut, B. , van den Beek, M. , Bouvier, D. , Cech, M. , Chilton, J. , Clements, D. , Coraor, N. , Grüning, B. A. , Guerler, A. , Hillman‐Jackson, J. , Hiltemann, S. , Jalili, V. , Rasche, H. , Soranzo, N. , Goecks, J. , Taylor, J. , Nekrutenko, A. , & Blankenberg, D. (2018). The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research, 46, W537–w544. 10.1093/nar/gky379 - DOI - PMC - PubMed
-
- Armani, A. , Galli, S. , Giacomello, E. , Bagnato, P. , Barone, V. , Rossi, D. , & Sorrentino, V. (2006). Molecular interactions with obscurin are involved in the localization of muscle‐specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Experimental Cell Research, 312, 3546–3558. 10.1016/j.yexcr.2006.07.027 - DOI - PubMed
-
- Avelar, R. A. , Ortega, J. G. , Tacutu, R. , Tyler, E. J. , Bennett, D. , Binetti, P. , Budovsky, A. , Chatsirisupachai, K. , Johnson, E. , Murray, A. , Shields, S. , Tejada‐Martinez, D. , Thornton, D. , Fraifeld, V. E. , Bishop, C. L. , & de Magalhães, J. P. (2020). A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biology, 21, 91. 10.1186/s13059-020-01990-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

