Excessive tubulin glutamylation leads to progressive cone-rod dystrophy and loss of outer segment integrity
- PMID: 38297980
- PMCID: PMC12099296
- DOI: 10.1093/hmg/ddae013
Excessive tubulin glutamylation leads to progressive cone-rod dystrophy and loss of outer segment integrity
Abstract
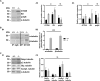
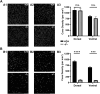
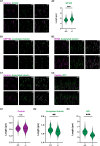
Mutations in Cytosolic Carboxypeptidase-like Protein 5 (CCP5) are associated with vision loss in humans. To decipher the mechanisms behind CCP5-associated blindness, we generated a novel mouse model lacking CCP5. In this model, we found that increased tubulin glutamylation led to progressive cone-rod dystrophy, with cones showing a more pronounced and earlier functional loss than rod photoreceptors. The observed functional reduction was not due to cell death, levels, or the mislocalization of major phototransduction proteins. Instead, the increased tubulin glutamylation caused shortened photoreceptor axonemes and the formation of numerous abnormal membranous whorls that disrupted the integrity of photoreceptor outer segments (OS). Ultimately, excessive tubulin glutamylation led to the progressive loss of photoreceptors, affecting cones more severely than rods. Our results highlight the importance of maintaining tubulin glutamylation for normal photoreceptor function. Furthermore, we demonstrate that murine cone photoreceptors are more sensitive to disrupted tubulin glutamylation levels than rods, suggesting an essential role for axoneme in the structural integrity of the cone outer segment. This study provides valuable insights into the mechanisms of photoreceptor diseases linked to excessive tubulin glutamylation.
Keywords: blindness; photoreceptors; protein posttranslational modification; retinal degeneration; tubulin modification.
© The Author(s) 2024. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures







References
-
- Walaas SI, Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev 1991;43:299–349. - PubMed
-
- Edde B, Rossier J, Le Caer JP. et al. Posttranslational glutamylation of alpha-tubulin. Science 1990;247:83–5. - PubMed
-
- McKenna ED, Sarbanes SL, Cummings SW. et al. The tubulin code, from molecules to health and disease. Annu Rev Cell Dev Biol 2023;39:331–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

