Diverse drug delivery systems for the enhancement of cancer immunotherapy: an overview
- PMID: 38298192
- PMCID: PMC10828056
- DOI: 10.3389/fimmu.2024.1328145
Diverse drug delivery systems for the enhancement of cancer immunotherapy: an overview
Abstract
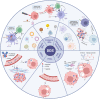
Despite the clear benefits demonstrated by immunotherapy, there is still an inevitable off-target effect resulting in serious adverse immune reactions. In recent years, the research and development of Drug Delivery System (DDS) has received increased prominence. In decades of development, DDS has demonstrated the ability to deliver drugs in a precisely targeted manner to mitigate side effects and has the advantages of flexible control of drug release, improved pharmacokinetics, and drug distribution. Therefore, we consider that combining cancer immunotherapy with DDS can enhance the anti-tumor ability. In this paper, we provide an overview of the latest drug delivery strategies in cancer immunotherapy and briefly introduce the characteristics of DDS based on nano-carriers (liposomes, polymer nano-micelles, mesoporous silica, extracellular vesicles, etc.) and coupling technology (ADCs, PDCs and targeted protein degradation). Our aim is to show readers a variety of drug delivery platforms under different immune mechanisms, and analyze their advantages and limitations, to provide more superior and accurate targeting strategies for cancer immunotherapy.
Keywords: cancer immunotherapy; coupled drugs; drug delivery; multidrug combination; nanoparticles; protein degradation.
Copyright © 2024 Liu, Cheng, Mu, Zhang, Tian, Liu, Hu and Wen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

