Multi-epitopes vaccine design for surface glycoprotein against SARS-CoV-2 using immunoinformatic approach
- PMID: 38298616
- PMCID: PMC10827691
- DOI: 10.1016/j.heliyon.2024.e24186
Multi-epitopes vaccine design for surface glycoprotein against SARS-CoV-2 using immunoinformatic approach
Abstract
Background: The recent COVID vaccinations have successfully reduced death and severity but did not stop the transmission of viruses by the emerging SARS-CoV-2 strain. There is a need for better and long-lasting dynamic vaccines for numerous prevailing strains and the evolving SARS-CoV-2 virus, necessitating the development of broad-spectrum strains being used to stop infection by reducing the spread rate and re-infection. The spike (S) glycoprotein is one of the proteins expressed commonly in the early phases of SARS-CoV-2 infection. It has been identified as the most immunogenic protein of SARS-CoV-2.
Methods: In this study, advanced bioinformatics techniques have been exploited to design the novel multi-epitope vaccine using conserved S protein portions from widespread strains of SARS-CoV-2 to predict B cell and T cell epitopes. These epitopes were selected based on toxicity, antigenicity score and immunogenicity. Epitope combinations were used to construct the maximum potent multi-epitope construct with potential immunogenic features. EAAAK, AAY, and GPGPG were used as linkers to construct epitopes.
Results: The developed vaccine has shown positive results. After the chimeric vaccine construct was cloned into the PET28a (+) vector for expression screening in Escherichia coli, the potential expression of the construct was identified.
Conclusion: The construct vaccine performed well in computer-based immune response simulation and covered a variety of allelic populations. These computational results are more helpful for further analysis of our contract vaccine, which can finally help control and prevent SARS-CoV-2 infections worldwide.
Keywords: Antigenicity; B cell; Construct; Epitopes; Immune; Prediction; T cell; Vector.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


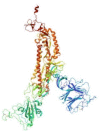



References
-
- Al-Qahtani W.S., Alneghery L.M., Alqahtani A.Q.S., Alkahtani M.D., Alkahtani S. A review of comparison study between corona viruses (Sars-cov, mers-cov) and novel corona virus (COVID-19) Rev. Mex. Ing. Quim. 2020;19:201–212. doi: 10.24275/rmiq/Bio1692. - DOI
-
- Rabaan A.A., Al-Ahmed S.H., Haque S., Sah R., Tiwari R., Malik Y.S., Dhama K., Yatoo M.I., Bonilla-Aldana D.K., Rodriguez-Morales A.J. SARS-CoV-2, SARS-CoV, and MERS-CoV: a comparative overview. Infez. Med. 2020;28:174–184. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

