Integrating network pharmacology and experimental verification to decipher the multitarget pharmacological mechanism of Cinnamomum zeylanicum essential oil in treating inflammation
- PMID: 38298712
- PMCID: PMC10828654
- DOI: 10.1016/j.heliyon.2024.e24120
Integrating network pharmacology and experimental verification to decipher the multitarget pharmacological mechanism of Cinnamomum zeylanicum essential oil in treating inflammation
Abstract
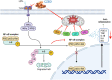
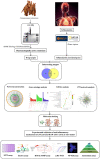
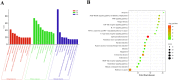
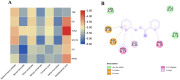
Inflammatory diseases contribute to more than 50 % of global deaths. Research suggests that network pharmacology can reveal the biological mechanisms underlying inflammatory diseases and drug effects at the molecular level. The aim of the study was to clarify the biological mechanism of Cinnamomum zeylanicum essential oil (CZEO) and predict molecular targets of CZEO against inflammation by employing network pharmacology and in vitro assays. First, the genes related to inflammation were identified from the Genecards and Online Mendelian Inheritance in Man (OMIM) databases. The CZEO targets were obtained from the SwissTargetPrediction and Similarity Ensemble Approach (SEA) database. A total of 1057 CZEO and 526 anti-inflammation targets were obtained. The core hub target of CZEO anti-inflammatory was obtained using the protein-protein interaction network. KEGG pathway analysis suggested CZEO to exert anti-inflammatory effect mainly through Tumor necrosis factor, Toll-like receptor and IL-17 signalling pathway. Molecular docking of active ingredients-core targets interactions was modelled using Pyrx software. Docking and simulation studies revealed benzyl benzoate to exhibit good binding affinity towards IL8 protein. MTT assay revealed CZEO to have non-cytotoxic effect on RAW 264.7 cells. CZEO also inhibited the production of NO, PGE2, IL-6, IL-1β and TNF-α and promoted the activity of endogenous antioxidant enzymes in LPS-stimulated RAW 264.7 cells. Additionally, CZEO inhibited intracellular ROS generation, NF-kB nuclear translocation and modulated the expression of downstream genes involved in Toll-like receptor signalling pathway. The results deciphered the mechanism of CZEO in treating inflammation and provided a theoretical basis for its clinical application.
Keywords: Anti-inflammatory; Cinnamomum zeylanicum bark essential oil; Molecular docking; Network pharmacology; RAW 264.7 cells.
© 2024 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:No If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Integrative approach to decipher pharmacological mechanism of Cinnamomum zeylanicum essential oil in prostate cancer.Med Oncol. 2025 Mar 12;42(4):100. doi: 10.1007/s12032-025-02665-w. Med Oncol. 2025. PMID: 40072751
-
Exploration of Pharmacological Mechanism of Cinnamomum tamala Essential Oil in Treating Inflammation based on Network Pharmacology, Molecular Modelling, and Experimental Validation.Curr Pharm Des. 2024;30(37):2959-2977. doi: 10.2174/0113816128342075240816104654. Curr Pharm Des. 2024. PMID: 39171467
-
Integrating network pharmacology, molecular docking and experimental verification to reveal the mechanism of artesunate in inhibiting choroidal melanoma.Front Pharmacol. 2024 Aug 9;15:1448381. doi: 10.3389/fphar.2024.1448381. eCollection 2024. Front Pharmacol. 2024. PMID: 39185308 Free PMC article.
-
Investigation of the Molecular Mechanisms Underlying the Therapeutic Effect of Perilla frutescens L. Essential Oil on Acute Lung Injury Using Gas Chromatography-Mass Spectrometry and Network Pharmacology.Comb Chem High Throughput Screen. 2024;27(10):1480-1494. doi: 10.2174/0113862073244521231003071900. Comb Chem High Throughput Screen. 2024. PMID: 37818572
-
The effect of Cinnamomum zeylanicum essential oil on chemical characteristics of Lyoner- type sausage during refrigerated storage.Vet Res Forum. 2015 Winter;6(1):31-9. Epub 2015 Mar 15. Vet Res Forum. 2015. PMID: 25992249 Free PMC article.
Cited by
-
Molecular docking and network pharmacology study on active compounds of Cyprus rotundus for the treatment of diabetes mellitus.In Silico Pharmacol. 2024 Nov 2;12(2):98. doi: 10.1007/s40203-024-00273-6. eCollection 2024. In Silico Pharmacol. 2024. PMID: 39498162
-
Elucidating the anti-cancer potential of Cinnamomum tamala essential oil against non-small cell lung cancer: A multifaceted approach involving GC-MS profiling, network pharmacology, and molecular dynamics simulations.Heliyon. 2024 Mar 16;10(6):e28026. doi: 10.1016/j.heliyon.2024.e28026. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38533033 Free PMC article.
-
Network pharmacology to explore the novel anti-inflammatory mechanism of naringenin in intestinal ischemia/reperfusion injury.Front Immunol. 2025 Aug 8;16:1623080. doi: 10.3389/fimmu.2025.1623080. eCollection 2025. Front Immunol. 2025. PMID: 40861465 Free PMC article.
-
Integrative approach to decipher pharmacological mechanism of Cinnamomum zeylanicum essential oil in prostate cancer.Med Oncol. 2025 Mar 12;42(4):100. doi: 10.1007/s12032-025-02665-w. Med Oncol. 2025. PMID: 40072751
-
Cinnamaldehyde-Mediated Suppression of MMP-13, COX-2, and IL-6 Through MAPK and NF-κB Signaling Inhibition in Chondrocytes and Synoviocytes Under Inflammatory Conditions.Int J Mol Sci. 2024 Nov 30;25(23):12914. doi: 10.3390/ijms252312914. Int J Mol Sci. 2024. PMID: 39684628 Free PMC article.
References
-
- El-Banna A.A., Darwish R.S., Ghareeb D.A., Yassin A.M., Abdulmalek S.A., Dawood H.M. Metabolic profiling of Lantana camara L. using UPLC-MS/MS and revealing its inflammation-related targets using network pharmacology-based and molecular docking analyses. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-19137-0. - DOI - PMC - PubMed
-
- Williams L.B., Koenig J.B., Black B., Gibson T.W.G., Sharif S., Koch T.G. Equine allogeneic umbilical cord blood derived mesenchymal stromal cells reduce synovial fluid nucleated cell count and induce mild self‐limiting inflammation when evaluated in an lipopolysaccharide induced synovitis model. Equine Vet. J. 2016;48:619–625. doi: 10.1111/evj.12477. - DOI - PubMed
LinkOut - more resources
Full Text Sources

