Neutrophils and Neutrophil Extracellular Traps Cause Vascular Occlusion and Delayed Cerebral Ischemia After Subarachnoid Hemorrhage in Mice
- PMID: 38299355
- PMCID: PMC10923061
- DOI: 10.1161/ATVBAHA.123.320224
Neutrophils and Neutrophil Extracellular Traps Cause Vascular Occlusion and Delayed Cerebral Ischemia After Subarachnoid Hemorrhage in Mice
Abstract
Background: After subarachnoid hemorrhage (SAH), neutrophils are deleterious and contribute to poor outcomes. Neutrophils can produce neutrophil extracellular traps (NETs) after ischemic stroke. Our hypothesis was that, after SAH, neutrophils contribute to delayed cerebral ischemia (DCI) and worse outcomes via cerebrovascular occlusion by NETs.
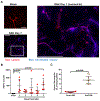
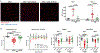
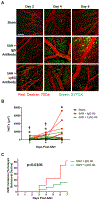
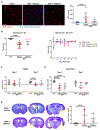
Methods: SAH was induced via endovascular perforation, and SAH mice were given either a neutrophil-depleting antibody, a PAD4 (peptidylarginine deiminase 4) inhibitor (to prevent NETosis), DNAse-I (to degrade NETs), or a vehicle control. Mice underwent daily neurological assessment until day 7 and then euthanized for quantification of intravascular brain NETs (iNETs). Subsets of mice were used to quantify neutrophil infiltration, NETosis potential, iNETs, cerebral perfusion, and infarction. In addition, NET markers were assessed in the blood of aneurysmal SAH patients.
Results: In mice, SAH led to brain neutrophil infiltration within 24 hours, induced a pro-NETosis phenotype selectively in skull neutrophils, and caused a significant increase in iNETs by day 1, which persisted until at least day 7. Neutrophil depletion significantly reduced iNETs, improving cerebral perfusion, leading to less neurological deficits and less incidence of DCI (16% versus 51.9%). Similarly, PAD4 inhibition reduced iNETs, improved neurological outcome, and reduced incidence of DCI (5% versus 30%), whereas degrading NETs marginally improved outcomes. Patients with aneurysmal SAH who developed DCI had elevated markers of NETs compared with non-DCI patients.
Conclusions: After SAH, skull-derived neutrophils are primed for NETosis, and there are persistent brain iNETs, which correlated with delayed deficits. The findings from this study suggest that, after SAH, neutrophils and NETosis are therapeutic targets, which can prevent vascular occlusion by NETs in the brain, thereby lessening the risk of DCI. Finally, NET markers may be biomarkers, which can predict which patients with aneurysmal SAH are at risk for developing DCI.
Keywords: biomarkers; brain; delayed cerebral ischemia; neutrophil extracellular traps; subarachnoid hemorrhage.
Conflict of interest statement
Figures





References
-
- Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–37. - PubMed
-
- Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J and Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth. 2012;109:315–29. - PubMed
-
- Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

