The intersection of host in vivo metabolism and immune responses to infection with kinetoplastid and apicomplexan parasites
- PMID: 38299836
- PMCID: PMC10966954
- DOI: 10.1128/mmbr.00164-22
The intersection of host in vivo metabolism and immune responses to infection with kinetoplastid and apicomplexan parasites
Abstract
SUMMARYProtozoan parasite infection dramatically alters host metabolism, driven by immunological demand and parasite manipulation strategies. Immunometabolic checkpoints are often exploited by kinetoplastid and protozoan parasites to establish chronic infection, which can significantly impair host metabolic homeostasis. The recent growth of tools to analyze metabolism is expanding our understanding of these questions. Here, we review and contrast host metabolic alterations that occur in vivo during infection with Leishmania, trypanosomes, Toxoplasma, Plasmodium, and Cryptosporidium. Although genetically divergent, there are commonalities among these pathogens in terms of metabolic needs, induction of the type I immune responses required for clearance, and the potential for sustained host metabolic dysbiosis. Comparing these pathogens provides an opportunity to explore how transmission strategy, nutritional demand, and host cell and tissue tropism drive similarities and unique aspects in host response and infection outcome and to design new strategies to treat disease.
Keywords: Cryptosporidium; Leishmania; Plasmodium; Toxoplasma gondii; Trypanosoma; apicomplexan; host-parasite relationship; immunity; kinetoplastida; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
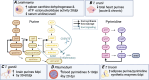
Figures



References
-
- Jara M, Arevalo J, Llanos-Cuentas A, den Broeck FV, Domagalska MA, Dujardin J-C. 2023. Unveiling drug-tolerant and persister-like cells in Leishmania braziliensis lines derived from patients with cutaneous leishmaniasis. Front Cell Infect Microbiol 13:1253033. doi:10.3389/fcimb.2023.1253033 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

